| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:47:14 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034259 |
|---|
| Identification |
|---|
| Common Name | Heterophylol |
|---|
| Class | Small Molecule |
|---|
| Description | Heterophylol is found in fruits. Heterophylol is a constituent of the root bark of Artocarpus heterophyllus (jackfruit). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
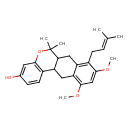
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6a,7,12,12a-tetrahydro-9,11-Dimethoxy-6,6-dimethyl-8-(3-methyl-2-butenyl)-6H-benzo[b]naphtho[2,3-D]pyran-3-ol, 9ci | HMDB |
|
|---|
| Chemical Formula | C26H32O4 |
|---|
| Average Molecular Mass | 408.530 g/mol |
|---|
| Monoisotopic Mass | 408.230 g/mol |
|---|
| CAS Registry Number | 152841-81-1 |
|---|
| IUPAC Name | 9,11-dimethoxy-6,6-dimethyl-8-(3-methylbut-2-en-1-yl)-6a,7,12,12a-tetrahydro-6H-5-oxatetraphen-3-ol |
|---|
| Traditional Name | 9,11-dimethoxy-6,6-dimethyl-8-(3-methylbut-2-en-1-yl)-6a,7,12,12a-tetrahydro-5-oxatetraphen-3-ol |
|---|
| SMILES | COC1=CC(OC)=C2CC3C(CC2=C1CC=C(C)C)C(C)(C)OC1=C3C=CC(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C26H32O4/c1-15(2)7-9-17-19-13-22-20(12-21(19)24(29-6)14-23(17)28-5)18-10-8-16(27)11-25(18)30-26(22,3)4/h7-8,10-11,14,20,22,27H,9,12-13H2,1-6H3 |
|---|
| InChI Key | VDNFSSVVXBUKRX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthopyran
- 2,2-dimethyl-1-benzopyran
- Chromane
- Benzopyran
- 1-benzopyran
- Tetralin
- Naphthalene
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Pyran
- Ether
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-1149000000-c2a0073e7b0316a5ba11 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-014i-2041900000-af7de81e7707316cbf56 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0016900000-d70570213dcb6e1dcc40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066r-1159100000-1b81804d146d7fdf9e52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-7196000000-50b6d67a7f7e9bbe6cf2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0001900000-20e1048f1f71db82967a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0009800000-3a3081a048efdfbc879a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03k9-1009000000-24a57a9a8174e85ea4da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pb9-0007900000-93886434121dc693a272 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-0196300000-5c7d6b776a32bd4ef174 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0r0d-1039000000-bdc92ecf5599358322c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-150cf3642e69ed410a9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0005900000-7c0f4a5f79e3a3344e79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-0009100000-52b17b5c0e376d23dca7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041126 |
|---|
| FooDB ID | FDB021008 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057918 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015111 |
|---|
| ChEBI ID | 176014 |
|---|
| PubChem Compound ID | 15227962 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|