| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:47:11 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034258 |
|---|
| Identification |
|---|
| Common Name | Artonin R |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from Artocarpus heterophyllus (jackfruit). Artonin R is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
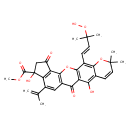
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 21-[(1E)-3-hydroperoxy-3-methylbut-1-en-1-yl]-7,14-dihydroxy-18,18-dimethyl-5,12-dioxo-9-(prop-1-en-2-yl)-2,19-dioxapentacyclo[11.8.0.0³,¹¹.0⁴,⁸.0¹⁵,²⁰]henicosa-1(13),3(11),4(8),9,14,16,20-heptaene-7-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C31H30O10 |
|---|
| Average Molecular Mass | 562.564 g/mol |
|---|
| Monoisotopic Mass | 562.184 g/mol |
|---|
| CAS Registry Number | 161017-01-2 |
|---|
| IUPAC Name | methyl 21-[(1E)-3-hydroperoxy-3-methylbut-1-en-1-yl]-7,14-dihydroxy-18,18-dimethyl-5,12-dioxo-9-(prop-1-en-2-yl)-2,19-dioxapentacyclo[11.8.0.0³,¹¹.0⁴,⁸.0¹⁵,²⁰]henicosa-1(13),3(11),4(8),9,14,16,20-heptaene-7-carboxylate |
|---|
| Traditional Name | methyl 21-[(1E)-3-hydroperoxy-3-methylbut-1-en-1-yl]-7,14-dihydroxy-18,18-dimethyl-5,12-dioxo-9-(prop-1-en-2-yl)-2,19-dioxapentacyclo[11.8.0.0³,¹¹.0⁴,⁸.0¹⁵,²⁰]henicosa-1(13),3(11),4(8),9,14,16,20-heptaene-7-carboxylate |
|---|
| SMILES | COC(=O)C1(O)CC(=O)C2=C1C(=CC1=C2OC2=C(C(O)=C3C=CC(C)(C)OC3=C2\C=C\C(C)(C)OO)C1=O)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C31H30O10/c1-14(2)17-12-18-24(34)21-23(33)15-8-10-29(3,4)40-25(15)16(9-11-30(5,6)41-37)26(21)39-27(18)20-19(32)13-31(36,22(17)20)28(35)38-7/h8-12,33,36-37H,1,13H2,2-7H3/b11-9+ |
|---|
| InChI Key | DHSZUELPJXKISQ-PKNBQFBNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranoxanthones. These are organic aromatic compounds containing a pyran or a hydrogenated derivative fused to a xanthone ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Pyranoxanthones |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-1000390000-6645d7e65b82eef49268 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00yi-9000547000-9e2320d4f0d2dd878f93 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Artonin R,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-1000390000-c90ab2c49d8d336a9a3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-024i-3000890000-6930ceafa3e1eca7791b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2000900000-33887f4581ed4e00b568 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-7edfaf126f57bc451fad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000490000-62c1b4736326e2b2e566 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-1010900000-9a9cc245f8e83a1fe9f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000910000-859efc036797841ba813 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000940000-36b341474849b893e7cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4s-1002930000-a2e7dd09d8709e585c32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0000290000-c26a453f890186918a29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00b9-1000950000-c0a5c4400560f10a0504 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01t9-2000920000-0aaccc676385512e1726 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041125 |
|---|
| FooDB ID | FDB021007 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131753035 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|