| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:47:06 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034256 |
|---|
| Identification |
|---|
| Common Name | Kinobeon A |
|---|
| Class | Small Molecule |
|---|
| Description | Kinobeon A is found in fats and oils. Kinobeon A is a pigment produced by cultures of Carthamus tinctorius (safflower). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
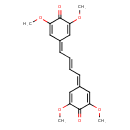
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-Bis(3,5-dimethoxy-4-oxo-2,5-cyclohexadienylidene)-2-butene | HMDB | | 4,4'-(2-Butene-1,4-diylidene)bis(2,6-dimethoxy-2,5-cyclohexadien-1-one), 9ci | HMDB | | Kinobeon a | MeSH |
|
|---|
| Chemical Formula | C20H20O6 |
|---|
| Average Molecular Mass | 356.369 g/mol |
|---|
| Monoisotopic Mass | 356.126 g/mol |
|---|
| CAS Registry Number | 155239-87-5 |
|---|
| IUPAC Name | 4-[(2E)-4-(3,5-dimethoxy-4-oxocyclohexa-2,5-dien-1-ylidene)but-2-en-1-ylidene]-2,6-dimethoxycyclohexa-2,5-dien-1-one |
|---|
| Traditional Name | 4-[(2E)-4-(3,5-dimethoxy-4-oxocyclohexa-2,5-dien-1-ylidene)but-2-en-1-ylidene]-2,6-dimethoxycyclohexa-2,5-dien-1-one |
|---|
| SMILES | COC1=CC(=C\C=C\C=C2C=C(OC)C(=O)C(OC)=C2)C=C(OC)C1=O |
|---|
| InChI Identifier | InChI=1S/C20H20O6/c1-23-15-9-13(10-16(24-2)19(15)21)7-5-6-8-14-11-17(25-3)20(22)18(12-14)26-4/h5-12H,1-4H3/b6-5+ |
|---|
| InChI Key | KQTQOJWCKLPTGL-AATRIKPKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as p-quinomethanes. These are organic compounds containing a benzene ring conjugated to a methylidene group and a ketone at carbon atoms 1 and 4, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | P-quinomethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-quinomethane
- Organic oxide
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056r-1269000000-48ae8f1e68a0c6a4bbaa | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-747c619b97d8f379f962 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ar0-0569000000-da79c0dc1be6cb384e74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f92-9542000000-3b101b664137a2fc94d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-1ed59a5bdd21e1919ad8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0009000000-321c7c981d513ee8836a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-1089000000-d69c0c3ecf9c58592edd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0029000000-083c044a424be2425632 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0069000000-4648edf4b6e6c20d6d64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-1092000000-af47788ffdc46f6cac2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-589d763c674c502bf315 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-0049000000-0979727c161c0ff85a20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-0097000000-b07cdf3be39883e99741 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041123 |
|---|
| FooDB ID | FDB021005 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055447 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8412545 |
|---|
| ChEBI ID | 175575 |
|---|
| PubChem Compound ID | 10237057 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|