| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:45:59 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034233 |
|---|
| Identification |
|---|
| Common Name | Kanzonol R |
|---|
| Class | Small Molecule |
|---|
| Description | Kanzonol R is found in herbs and spices. Kanzonol R is a constituent of Glycyrrhiza glabra (licorice). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
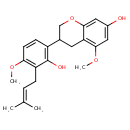
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3R)-7,2'-Dihydroxy-5,4'-dimethoxy-3'-prenylisoflavan | HMDB | | 2',7-Dihydroxy-4',5-dimethoxy-3'-prenylisoflavan | HMDB |
|
|---|
| Chemical Formula | C22H26O5 |
|---|
| Average Molecular Mass | 370.439 g/mol |
|---|
| Monoisotopic Mass | 370.178 g/mol |
|---|
| CAS Registry Number | 156250-73-6 |
|---|
| IUPAC Name | 3-[2-hydroxy-4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]-5-methoxy-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| Traditional Name | 3-[2-hydroxy-4-methoxy-3-(3-methylbut-2-en-1-yl)phenyl]-5-methoxy-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| SMILES | COC1=C(CC=C(C)C)C(O)=C(C=C1)C1COC2=CC(O)=CC(OC)=C2C1 |
|---|
| InChI Identifier | InChI=1S/C22H26O5/c1-13(2)5-6-17-19(25-3)8-7-16(22(17)24)14-9-18-20(26-4)10-15(23)11-21(18)27-12-14/h5,7-8,10-11,14,23-24H,6,9,12H2,1-4H3 |
|---|
| InChI Key | RRBCXJUMJUPDST-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 5-o-methylated isoflavonoids. These are isoflavonoids with methoxy groups attached to the C5 atom of the isoflavonoid backbone. Isoflavonoids are natural products derived from 3-phenylchromen-4-one. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | O-methylated isoflavonoids |
|---|
| Direct Parent | 5-O-methylated isoflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4p-methoxyisoflavonoid
- 5-methoxyisoflavonoid-skeleton
- Hydroxyisoflavonoid
- Isoflavanol
- Isoflavan
- Chromane
- Benzopyran
- Methoxyphenol
- 1-benzopyran
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-1029000000-d0876df14ce28ec3b586 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0f6t-2030920000-821ccf75b6599228f51a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0907000000-457f5ef0ea037ec36a2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zg0-3923000000-8bc7d22ecfd450620358 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-8903000000-7d789d708b8df575b3da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0209000000-9cabee3d4faaa38d6132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fvi-0907000000-e41af49874561407e735 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0935000000-5e7b760805e4fb3770da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0209000000-b875099ea9ea4cc6a724 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00li-0903000000-68e97478783bdaf08b32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3915000000-b65febecc1b707b7edc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-bc94bef3adca75259385 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-0009000000-fe55a9e3512219049d11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-084i-1729000000-c1dca8165789c4b9b3f5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041099 |
|---|
| FooDB ID | FDB020981 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019330 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015103 |
|---|
| ChEBI ID | 175772 |
|---|
| PubChem Compound ID | 131753027 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|