| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:45:37 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034225 |
|---|
| Identification |
|---|
| Common Name | 1-(4-Hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-3,5-heptanediol |
|---|
| Class | Small Molecule |
|---|
| Description | 1-(4-Hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-3,5-heptanediol is found in herbs and spices. 1-(4-Hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-3,5-heptanediol is a constituent of the dried rhizomes of Zingiber officinale (ginger). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
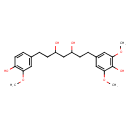
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,5-Dihydroxy-meodah | HMDB | | 3,5-Dihydroxy-1-(4'-hydroxy-3',5'-dimethoxyphenyl)-7-(4''-hydroxy-3''-methoxyphenyl)heptane | MeSH |
|
|---|
| Chemical Formula | C22H30O7 |
|---|
| Average Molecular Mass | 406.469 g/mol |
|---|
| Monoisotopic Mass | 406.199 g/mol |
|---|
| CAS Registry Number | 145888-84-2 |
|---|
| IUPAC Name | 1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-diol |
|---|
| Traditional Name | 1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane-3,5-diol |
|---|
| SMILES | COC1=CC(CCC(O)CC(O)CCC2=CC(OC)=C(O)C=C2)=CC(OC)=C1O |
|---|
| InChI Identifier | InChI=1S/C22H30O7/c1-27-19-10-14(6-9-18(19)25)4-7-16(23)13-17(24)8-5-15-11-20(28-2)22(26)21(12-15)29-3/h6,9-12,16-17,23-26H,4-5,7-8,13H2,1-3H3 |
|---|
| InChI Key | UEKHBUNMFZUBFK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as curcuminoids. These are aromatic compounds containing a curcumin moiety, which is composed of two aryl buten-2-one (feruloyl) chromophores joined by a methylene group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Diarylheptanoids |
|---|
| Sub Class | Linear diarylheptanoids |
|---|
| Direct Parent | Curcuminoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Curcumin
- Gingerdiol
- Methoxyphenol
- Dimethoxybenzene
- M-dimethoxybenzene
- Fatty alcohol
- Anisole
- Methoxybenzene
- Phenol ether
- Phenoxy compound
- Alkyl aryl ether
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Fatty acyl
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- Ether
- Alcohol
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0942000000-f8b499855888a6258aba | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-004i-4023309000-9381bcb7725b2f4457d0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0109300000-1d4491da4952b9b3483c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0947000000-1e870cbb04556bcd9a81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fri-1932000000-6705b3f249ff846ae685 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0003900000-6716a7e0107793068bdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a70-0539200000-cdb0dd8e26409d81b81d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-0539000000-ddfe0afe657b9effb820 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0109200000-bb21e0c4aebcce8d556d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0953000000-b37c06b46b6c3c503e05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0932000000-b2115c6fa523708e48a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0012900000-0361e7a9b8434a5b11af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-0249100000-5a70bc1c667cb4c8a190 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ldi-1349000000-c52055b9ad5e5779207a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041091 |
|---|
| FooDB ID | FDB020969 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 23254830 |
|---|
| ChEBI ID | 176008 |
|---|
| PubChem Compound ID | 53462009 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|