| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:45:11 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034216 |
|---|
| Identification |
|---|
| Common Name | (5Z,8Z)-1,5,8-Heptadecatriene |
|---|
| Class | Small Molecule |
|---|
| Description | (5Z,8Z)-1,5,8-Heptadecatriene is found in tea. (5Z,8Z)-1,5,8-Heptadecatriene is a constituent of the essential oil of Tussilago farfara (coltsfoot). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
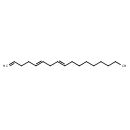
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C17H30 |
|---|
| Average Molecular Mass | 234.420 g/mol |
|---|
| Monoisotopic Mass | 234.235 g/mol |
|---|
| CAS Registry Number | 145297-96-7 |
|---|
| IUPAC Name | (5E,8E)-heptadeca-1,5,8-triene |
|---|
| Traditional Name | (5E,8E)-heptadeca-1,5,8-triene |
|---|
| SMILES | CCCCCCCC\C=C\C\C=C\CCC=C |
|---|
| InChI Identifier | InChI=1S/C17H30/c1-3-5-7-9-11-13-15-17-16-14-12-10-8-6-4-2/h3,9,11,15,17H,1,4-8,10,12-14,16H2,2H3/b11-9+,17-15+ |
|---|
| InChI Key | MYHCPULNJBZGRM-HFPDKQRBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkatrienes. These are acyclic hydrocarbons that contain exactly three carbon-to-carbon double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Olefins |
|---|
| Direct Parent | Alkatrienes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkatriene
- Unsaturated aliphatic hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4s-9600000000-bd50c70c4da5e4124c0a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-c3a9ef51bcd447603b5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9770000000-35876b22369956027daf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-dab44a6148b8e54e9679 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-8619dfe1d16ffe8ae768 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-8fb6052b9464db1e3377 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00o3-4930000000-82b3a62d894cb62edabd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-1e57a98cc7f3d8408756 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-0b2033b64ed6c76beee8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007o-5930000000-033c5827fe8fe2c63a63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-3390000000-1e6efa6fcf36b8ff303f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0api-9310000000-3ab539b58cfbfb9f0972 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-9100000000-4ff853cf4fea2bdda473 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041082 |
|---|
| FooDB ID | FDB020958 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777532 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 101631554 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|