| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:44:17 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034197 |
|---|
| Identification |
|---|
| Common Name | 1,2-Dimethoxy-13-methyl-[1,3]benzodioxolo[5,6-c]phenanthridine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from root bark of Zanthoxylum simulans (Szechuan pepper). 1,2-Dimethoxy-13-methyl-[1,3]benzodioxolo[5,6-c]phenanthridine is found in herbs and spices and fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
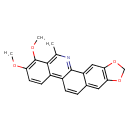
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Dimethoxy-13-methyl-[1,3]benzodioxolo[5,6-c]phenanthridine, 9ci | HMDB |
|
|---|
| Chemical Formula | C21H17NO4 |
|---|
| Average Molecular Mass | 347.364 g/mol |
|---|
| Monoisotopic Mass | 347.116 g/mol |
|---|
| CAS Registry Number | 154490-59-2 |
|---|
| IUPAC Name | 17,18-dimethoxy-20-methyl-5,7-dioxa-21-azapentacyclo[11.8.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁹]henicosa-1(21),2,4(8),9,11,13,15,17,19-nonaene |
|---|
| Traditional Name | 17,18-dimethoxy-20-methyl-5,7-dioxa-21-azapentacyclo[11.8.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁹]henicosa-1(21),2,4(8),9,11,13,15,17,19-nonaene |
|---|
| SMILES | COC1=C(OC)C2=C(C)N=C3C4=CC5=C(OCO5)C=C4C=CC3=C2C=C1 |
|---|
| InChI Identifier | InChI=1S/C21H17NO4/c1-11-19-13(6-7-16(23-2)21(19)24-3)14-5-4-12-8-17-18(26-10-25-17)9-15(12)20(14)22-11/h4-9H,10H2,1-3H3 |
|---|
| InChI Key | FYDZPRHXTQYOIW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenanthridines and derivatives. These are polycyclic compounds containing a phenanthridine moiety, which is a tricyclic system with two benzene rings joined by a pyridine forming a non-linear skeleton. Hydrogenated derivatives are also included. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Benzoquinolines |
|---|
| Direct Parent | Phenanthridines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenanthridine
- Isoquinoline
- Naphthalene
- Benzodioxole
- Anisole
- Alkyl aryl ether
- Methylpyridine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Oxacycle
- Acetal
- Ether
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gb9-0029000000-abe6eb2419b70e30ccba | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-82a3b57c63701da68137 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0009000000-3989ca736a1ce10a3b02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-0096000000-aabef0c7ff20b52147c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-ad7f5607c28a620c9bb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-27451b00ec063015131d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0w90-0096000000-11c198dbd3c41a96fbca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-285d9e72a283daa290ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0009000000-87ce46586e97dc38bddf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-0049000000-2de0deac8d80b256132e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-2282db83d9508a2924b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-12261fd8f8ea293d1b5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0049000000-b11e461655ba7cf4106b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041063 |
|---|
| FooDB ID | FDB020938 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777530 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 101673185 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|