| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:43:56 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034189 |
|---|
| Identification |
|---|
| Common Name | Phytocassane B |
|---|
| Class | Small Molecule |
|---|
| Description | Phytocassane B is found in cereals and cereal products. Phytoalexin from Oryza sativa (rice). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
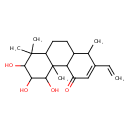
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C20H30O4 |
|---|
| Average Molecular Mass | 334.450 g/mol |
|---|
| Monoisotopic Mass | 334.214 g/mol |
|---|
| CAS Registry Number | 166547-22-4 |
|---|
| IUPAC Name | 2-ethenyl-5,6,7-trihydroxy-1,4b,8,8-tetramethyl-1,4,4a,4b,5,6,7,8,8a,9,10,10a-dodecahydrophenanthren-4-one |
|---|
| Traditional Name | 2-ethenyl-5,6,7-trihydroxy-1,4b,8,8-tetramethyl-4a,5,6,7,8a,9,10,10a-octahydro-1H-phenanthren-4-one |
|---|
| SMILES | CC1C2CCC3C(C)(C)C(O)C(O)C(O)C3(C)C2C(=O)C=C1C=C |
|---|
| InChI Identifier | InChI=1S/C20H30O4/c1-6-11-9-13(21)15-12(10(11)2)7-8-14-19(3,4)17(23)16(22)18(24)20(14,15)5/h6,9-10,12,14-18,22-24H,1,7-8H2,2-5H3 |
|---|
| InChI Key | XFRCVLKGZMPUFQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isocopalane and spongiane diterpenoids. These are diterpenoids with a structure based on the isocopalane (Tetradecahydro-1,1,4a,7,8,8a-hexamethylphenanthrene) or the 15,16-epoxyisocopalane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Isocopalane and spongiane diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isocopalane diterpenoid
- Hydrophenanthrene
- Phenanthrene
- Cyclohexenone
- Cyclitol or derivatives
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Polyol
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0g4i-0396000000-28b80391f7992bc18996 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-000i-1015690000-d49ba85c2067700c1b1c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0019000000-056d020ca31951393257 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gbj-5169000000-496188ac9ed471d839bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pvu-9181000000-e82d655e7f5629173b16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-a99700f3aaddf3302505 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0019000000-0089ea9241171fcd2c20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gba-1197000000-4d54429cb1681bab3743 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0019000000-df6088b2b17533a171f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-0193000000-637011017e43ebaacdc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wb9-4692000000-338533c89fa3ae72b395 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-fd5cd126f4d2eefd9030 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0019000000-cdb688693f1b5cbacf61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fsi-1279000000-d8b7e254b16e0d2e6f5f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041055 |
|---|
| FooDB ID | FDB020929 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034141 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24785736 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 85140086 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|