| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:43:54 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034188 |
|---|
| Identification |
|---|
| Common Name | Ustiloxin D |
|---|
| Class | Small Molecule |
|---|
| Description | Ustiloxin D is found in cereals and cereal products. Ustiloxin D is isolated from the false smut balls caused by Ustilaginoidea virens on rice. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
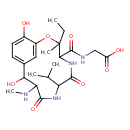
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-({[3-ethyl-6,9,11,15-tetrahydroxy-3-methyl-10-(methylamino)-7-(propan-2-yl)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(15),5,8,12(16),13-pentaen-4-yl](hydroxy)methylidene}amino)acetate | HMDB |
|
|---|
| Chemical Formula | C23H34N4O8 |
|---|
| Average Molecular Mass | 494.538 g/mol |
|---|
| Monoisotopic Mass | 494.238 g/mol |
|---|
| CAS Registry Number | 158243-18-6 |
|---|
| IUPAC Name | 2-{[3-ethyl-11,15-dihydroxy-3-methyl-10-(methylamino)-6,9-dioxo-7-(propan-2-yl)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),12,14-trien-4-yl]formamido}acetic acid |
|---|
| Traditional Name | {[3-ethyl-11,15-dihydroxy-7-isopropyl-3-methyl-10-(methylamino)-6,9-dioxo-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),12,14-trien-4-yl]formamido}acetic acid |
|---|
| SMILES | CCC1(C)OC2=CC(=CC=C2O)C(O)C(NC)C(=O)NC(C(C)C)C(=O)NC1C(=O)NCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C23H34N4O8/c1-6-23(4)19(22(34)25-10-15(29)30)27-20(32)16(11(2)3)26-21(33)17(24-5)18(31)12-7-8-13(28)14(9-12)35-23/h7-9,11,16-19,24,28,31H,6,10H2,1-5H3,(H,25,34)(H,26,33)(H,27,32)(H,29,30) |
|---|
| InChI Key | GDXLZSYACWZHOC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclic peptides. Cyclic peptides are compounds containing a cyclic moiety bearing a peptide backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Cyclic peptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclic alpha peptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Cyclic carboximidic acid
- Amino acid or derivatives
- Amino acid
- Secondary alcohol
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Polyol
- Azacycle
- Secondary amine
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Amine
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-9101500000-f4d35e0f4e95758e2d03 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-006x-9300044000-7be567447358c2ad5ee2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-1000900000-37b5e3246c5bf752172c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pdi-9302300000-8901e017fea61bb5199a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-9004100000-832fd979197d8e8bbf8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0001900000-53acda392808c38d90c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-4002900000-398bfbd6401dc72dc0ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03k9-5009000000-178a6144e3e6faa985be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-fa964fbaf366d8ea95e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0000900000-1e540b768274f4b2fd90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01tc-1009000000-5321a924f3750e93259d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0096-4000900000-27e69478f4253768dbe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0m4o-9307200000-65d074fcb590633202d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-024i-5009100000-22772fb757da6cf3fec1 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041054 |
|---|
| FooDB ID | FDB020928 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00016649 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015088 |
|---|
| ChEBI ID | 458649 |
|---|
| PubChem Compound ID | 72773471 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|