| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:43:49 UTC |
|---|
| Update Date | 2016-11-09 01:21:05 UTC |
|---|
| Accession Number | CHEM034186 |
|---|
| Identification |
|---|
| Common Name | Ajocysteine |
|---|
| Class | Small Molecule |
|---|
| Description | Ajocysteine is found in onion-family vegetables. Ajocysteine is a constituent of garlic (Allium sativum). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
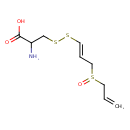
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-amino-4,5,9-Trithia-6,11-dodecadienoic acid 9-oxide | HMDB | | 2-Amino-3-{[(1E)-3-(prop-2-ene-1-sulfinyl)prop-1-en-1-yl]disulfanyl}propanoate | Generator | | 2-Amino-3-{[(1E)-3-(prop-2-ene-1-sulphinyl)prop-1-en-1-yl]disulphanyl}propanoate | Generator | | 2-Amino-3-{[(1E)-3-(prop-2-ene-1-sulphinyl)prop-1-en-1-yl]disulphanyl}propanoic acid | Generator |
|
|---|
| Chemical Formula | C9H15NO3S3 |
|---|
| Average Molecular Mass | 281.415 g/mol |
|---|
| Monoisotopic Mass | 281.021 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-amino-3-{[(1E)-3-(prop-2-ene-1-sulfinyl)prop-1-en-1-yl]disulfanyl}propanoic acid |
|---|
| Traditional Name | 2-amino-3-{[(1E)-3-(prop-2-ene-1-sulfinyl)prop-1-en-1-yl]disulfanyl}propanoic acid |
|---|
| SMILES | NC(CSS\C=C\CS(=O)CC=C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H15NO3S3/c1-2-5-16(13)6-3-4-14-15-7-8(10)9(11)12/h2-4,8H,1,5-7,10H2,(H,11,12)/b4-3+ |
|---|
| InChI Key | URBPKPSYQPHFAT-ONEGZZNKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cysteine and derivatives. Cysteine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Cysteine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cysteine or derivatives
- Alpha-amino acid
- Organic disulfide
- Allyl sulfur compound
- Amino acid
- Sulfoxide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Sulfenyl compound
- Sulfinyl compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9100000000-471065f4bf28b0b43454 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0005-9811000000-6e4308b2e6733c8c3dfd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0019-3290000000-c5454e9a6bd857492f2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000f-9620000000-7e8408e462c3a658e91c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-5d8c969f971a70146433 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0019-6490000000-fe0a5094384ede7df9f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9710000000-81bff71511a176589717 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000j-9200000000-305d5cbe93c4b6d3ea27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-3910000000-07aa4ea38d55a7473fcb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-9500000000-37b8a1cc6bc48cd5e779 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9100000000-7cd79b8f341db7efedf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0390000000-c12313a6f9aa118dec18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-9800000000-b2fc506747b255e418a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-9200000000-a4a2938ce640127a2db0 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041052 |
|---|
| FooDB ID | FDB020925 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015087 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 59288250 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|