| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:41:27 UTC |
|---|
| Update Date | 2016-11-09 01:21:04 UTC |
|---|
| Accession Number | CHEM034133 |
|---|
| Identification |
|---|
| Common Name | Ternatin heptapeptide |
|---|
| Class | Small Molecule |
|---|
| Description | "Ternatin" is a term used for two unrelated categories of biochemical compounds:
The ternatin heptapeptide — derived from the mushroom Coriolus versicolor
Delphinidin ternatins — derivatives of delphinidin, an anthocyanidin |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
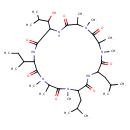
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4',5-Dihydroxy-3,3',7,8-tetramethoxy-flavone | MeSH | | Ternatin (flavonoid) | MeSH | | 4',5-Dihydroxy-3,3',7,8-tetramethoxyflavone | MeSH | | 5,4'-Dihydroxy-3,7,8,3'-tetramethoxyflavone | HMDB | | 5-Hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4H-1-benzopyran-4-one | HMDB | | cyclo(-OH-Leu-ile-(nme)ala-(nme)leu-leu-(nme)ala-(nme)ala-) | HMDB, MeSH | | Gossypetin 3,7,8,3'-tetramethyl ether | HMDB | | Ternatin a1 | HMDB | | Ternatin heptapeptide | HMDB | | cyclo(-OH-Leucyl-isoleucyl-(N-methyl)alanyl-(N-methyl)leucyl-leucyl-(N-methyl)alanyl-(N-methyl)alanyl-) | MeSH, HMDB |
|
|---|
| Chemical Formula | C37H67N7O8 |
|---|
| Average Molecular Mass | 737.970 g/mol |
|---|
| Monoisotopic Mass | 737.505 g/mol |
|---|
| CAS Registry Number | 148619-41-4 |
|---|
| IUPAC Name | 15-(butan-2-yl)-18-(1-hydroxy-2-methylpropyl)-1,3,4,10,12,13,21-heptamethyl-6,9-bis(2-methylpropyl)-1,4,7,10,13,16,19-heptaazacyclohenicosane-2,5,8,11,14,17,20-heptone |
|---|
| Traditional Name | 18-(1-hydroxy-2-methylpropyl)-1,3,4,10,12,13,21-heptamethyl-6,9-bis(2-methylpropyl)-15-(sec-butyl)-1,4,7,10,13,16,19-heptaazacyclohenicosane-2,5,8,11,14,17,20-heptone |
|---|
| SMILES | [H][C@@]1(NC(=O)[C@@H](C)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C)N(C)C(=O)[C@]([H])(NC1=O)[C@@H](C)CC)[C@H](O)C(C)C |
|---|
| InChI Identifier | InChI=1S/C37H67N7O8/c1-16-22(8)28-37(52)43(14)25(11)35(50)44(15)27(18-20(4)5)32(47)38-26(17-19(2)3)36(51)42(13)24(10)34(49)41(12)23(9)31(46)40-29(33(48)39-28)30(45)21(6)7/h19-30,45H,16-18H2,1-15H3,(H,38,47)(H,39,48)(H,40,46)/t22-,23+,24-,25-,26-,27-,28+,29+,30+/m0/s1 |
|---|
| InChI Key | ZMFVAIFXJWEOMH-PTPSPKLBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 8-o-methylated flavonoids. These are flavonoids with methoxy groups attached to the C8 atom of the flavonoid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | O-methylated flavonoids |
|---|
| Direct Parent | 8-O-methylated flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3p-methoxyflavonoid-skeleton
- 3-methoxyflavonoid-skeleton
- 7-methoxyflavonoid-skeleton
- 8-methoxyflavonoid-skeleton
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- Flavone
- Hydroxyflavonoid
- 3-methoxychromone
- Chromone
- Benzopyran
- Methoxyphenol
- 1-benzopyran
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Pyranone
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Pyran
- Benzenoid
- Monocyclic benzene moiety
- Vinylogous acid
- Heteroaromatic compound
- Organoheterocyclic compound
- Ether
- Oxacycle
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9000006400-4dce4913f2a9ad8ed9f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0000000900-55d2fd913ac031437936 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1000000900-a0e2db357cb8a93e02ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pi0-9000000300-c5275daf2469810dc38b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01p9-0000007900-c2417cafe903b15bf518 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fs-1000009300-d75f40ab7a6b5aec2478 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06r2-2000009100-e03f67f5ded4ce48b9da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000900-ec5cb3203a3d54046c14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000003900-0adf5a79207b65569f44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-074j-3000009000-6170bb2fa3566b43a0a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000000900-a2cf53b88b12af9c0781 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0000000900-0f870912f86bcad16e57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0nmi-1000009000-99e8ca322f55678f6be4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0145696 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00004737 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ternatin |
|---|
| Chemspider ID | 4573009 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5459184 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|