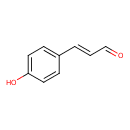
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-2900000000-886f27cd112d97db2407 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dj-6930000000-f587ab4886fa67b46bf3 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000t-0900000000-8d4db4516495a3b44081 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-53435f9a69270f05404d | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-000t-0900000000-55c5ad11349f59b1d018 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-0fwd-3900000000-97e3135074e2dfde1721 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-0fc0-6900000000-40696f3995cf56176be7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 6V, Positive | splash10-000t-0900000000-2e24a4894a1c8c280127 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0002-1900000000-33f5b65d20ab9f0c7fb6 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0fc0-6900000000-5f11bd25b21e3e2bc608 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000t-0900000000-e66a97c8c90f09504a25 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-b5bf04da75fd3c4f218d | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-bece2c2967ee385d8b9a | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0udj-3900000000-c4fb427576ce6d25c494 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-002b-0900000000-b62f984d6b010ca0b062 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0fwd-3900000000-0fff4b194cbbc9c167c1 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0f6t-3900000000-f9b7c3f6da276d1ce52f | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000t-0900000000-b0d12dfdd61803a9b0c1 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-c5f68610870a90b875f9 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0532-1900000000-da41d3b7e0fbce434380 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-9400000000-00d9adc10a5c57473a89 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-2da71b22701c15e439e5 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-0900000000-e1321c30ab9a5737d984 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00mo-5900000000-9c6281ba3b63cf35224e | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-d92260413651c4bb290d | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-49d5ed3a1403b3dbf32b | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-057i-9800000000-823da29e301d5a79f050 | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |