| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:38:50 UTC |
|---|
| Update Date | 2016-11-09 01:21:03 UTC |
|---|
| Accession Number | CHEM034079 |
|---|
| Identification |
|---|
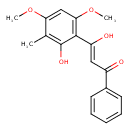
| Common Name | beta,2-Dihydroxy-4,6-dimethoxy-3-methylchalcone |
|---|
| Class | Small Molecule |
|---|
| Description | beta,2-Dihydroxy-4,6-dimethoxy-3-methylchalcone is found in tea. beta,2-Dihydroxy-4,6-dimethoxy-3-methylchalcone is a constituent of Leptospermum scoparium (red tea). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b,2-Dihydroxy-4,6-dimethoxy-3-methylchalcone | Generator | | Β,2-dihydroxy-4,6-dimethoxy-3-methylchalcone | Generator |
|
|---|
| Chemical Formula | C18H18O5 |
|---|
| Average Molecular Mass | 314.333 g/mol |
|---|
| Monoisotopic Mass | 314.115 g/mol |
|---|
| CAS Registry Number | 109845-46-7 |
|---|
| IUPAC Name | (2Z)-3-hydroxy-3-(2-hydroxy-4,6-dimethoxy-3-methylphenyl)-1-phenylprop-2-en-1-one |
|---|
| Traditional Name | (2Z)-3-hydroxy-3-(2-hydroxy-4,6-dimethoxy-3-methylphenyl)-1-phenylprop-2-en-1-one |
|---|
| SMILES | COC1=CC(OC)=C(\C(O)=C\C(=O)C2=CC=CC=C2)C(O)=C1C |
|---|
| InChI Identifier | InChI=1S/C18H18O5/c1-11-15(22-2)10-16(23-3)17(18(11)21)14(20)9-13(19)12-7-5-4-6-8-12/h4-10,20-21H,1-3H3/b14-9- |
|---|
| InChI Key | VDTALNHKFXTASW-ZROIWOOFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as retrochalcones. These are a form of normal chalcones that are structurally distinguished by the lack of oxygen functionalities at the C2'- and C6'-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Linear 1,3-diarylpropanoids |
|---|
| Sub Class | Chalcones and dihydrochalcones |
|---|
| Direct Parent | Retrochalcones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Retrochalcone
- Cinnamylphenol
- Hydroxycinnamic acid or derivatives
- Methoxyphenol
- Dimethoxybenzene
- M-dimethoxybenzene
- Anisole
- Benzoyl
- Phenoxy compound
- O-cresol
- Phenol ether
- Styrene
- Aryl ketone
- Methoxybenzene
- Toluene
- Alkyl aryl ether
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Enone
- Vinylogous acid
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Ketone
- Ether
- Enol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4j-0960000000-13b3f8ed80786dcae6ed | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0006-5205900000-0dd376d8cf58d077cc57 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05mk-0913000000-9341c7c1eb196a8fbbb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-0900000000-e2001b4665a65cd1a4ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2900000000-5b55f9007a5f181e3094 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0319000000-c618d7a2b10bc692a154 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0i01-0922000000-3b006341ea4a34d2cf3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-5930000000-17382cd4ecfd2185fb3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0409000000-553ec8692fe1b02dc2cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-1932000000-4531d26aacfbdf823693 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0v00-5940000000-a6598e908844468f3f3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0109000000-345a0727ef51ed8388f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066s-0900000000-7b60b479c064f7351393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-3900000000-1e2a9b9426e2138b04ca | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040936 |
|---|
| FooDB ID | FDB020777 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056445 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24838901 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|