| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:38:01 UTC |
|---|
| Update Date | 2016-11-09 01:21:03 UTC |
|---|
| Accession Number | CHEM034057 |
|---|
| Identification |
|---|
| Common Name | 2-(4-Methyl-1,3-pentadienyl)anthraquinone |
|---|
| Class | Small Molecule |
|---|
| Description | 2-(4-Methyl-1,3-pentadienyl)anthraquinone is found in fats and oils. 2-(4-Methyl-1,3-pentadienyl)anthraquinone is isolated from the hairy root culture of Sesamum indicum (sesame). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
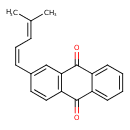
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(4-Methyl-1,3-pentadienyl)-9,10-anthracenedione, 9ci | HMDB | | 3-(3-((2-Methoxyethoxy)methoxy)-1-octenyl)cyclopentanone | HMDB |
|
|---|
| Chemical Formula | C20H16O2 |
|---|
| Average Molecular Mass | 288.340 g/mol |
|---|
| Monoisotopic Mass | 288.115 g/mol |
|---|
| CAS Registry Number | 150900-95-1 |
|---|
| IUPAC Name | 2-[(1Z)-4-methylpenta-1,3-dien-1-yl]-9,10-dihydroanthracene-9,10-dione |
|---|
| Traditional Name | 2-[(1Z)-4-methylpenta-1,3-dien-1-yl]anthracene-9,10-dione |
|---|
| SMILES | CC(C)=C\C=C/C1=CC2=C(C=C1)C(=O)C1=CC=CC=C1C2=O |
|---|
| InChI Identifier | InChI=1S/C20H16O2/c1-13(2)6-5-7-14-10-11-17-18(12-14)20(22)16-9-4-3-8-15(16)19(17)21/h3-12H,1-2H3/b7-5- |
|---|
| InChI Key | BIHNBOKNHXYFQJ-ALCCZGGFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthraquinones. These are organic compounds containing either anthracene-9,10-quinone, 1,4-anthraquinone, or 1,2-anthraquinone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthraquinones |
|---|
| Direct Parent | Anthraquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthraquinone
- 9,10-anthraquinone
- Aryl ketone
- Styrene
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0230-1690000000-65164dc31cca60850cdf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-fe649c849ba61544803d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0cdr-5790000000-bc86a4f4df66a936e025 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9440000000-0933a68d1fc040394ec5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-65f89d6ac8de37be5a35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-bb5d326a75090e813389 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2490000000-c3f6516b260ef28efbe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-c0e16271dbfb34967386 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-75abdeafe94f84240492 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fs-0190000000-060a826fbe9cdd3cdb50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-74bc6a0cff61652e4ea4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0090000000-fe213b6ace471e00dde7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ai-2490000000-c0bca1085e9a03a29c01 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040915 |
|---|
| FooDB ID | FDB020754 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 28285599 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 102387796 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|