| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:37:06 UTC |
|---|
| Update Date | 2016-11-09 01:21:03 UTC |
|---|
| Accession Number | CHEM034036 |
|---|
| Identification |
|---|
| Common Name | Ampelopsin D |
|---|
| Class | Small Molecule |
|---|
| Description | Ampelopsin D is found in alcoholic beverages. Ampelopsin D is a constituent of Vitis vinifera (wine grape). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
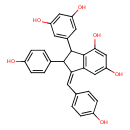
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+/-)-taxifolin | HMDB | | 3,3 ,4 ,5,7-Pentahydroxyflavonone | HMDB | | 3,3',4',5',7-Pentahydroxy-flavanone | HMDB | | 3,3',4',5,7-Pentahydroxy-flavanone | HMDB | | 3,3',4',5,7-Pentahydroxydihydroflavone | HMDB | | Ampelopsin | HMDB | | Dihydromyricetin | HMDB |
|
|---|
| Chemical Formula | C28H22O6 |
|---|
| Average Molecular Mass | 454.471 g/mol |
|---|
| Monoisotopic Mass | 454.142 g/mol |
|---|
| CAS Registry Number | 149418-37-1 |
|---|
| IUPAC Name | (1Z)-3-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)-1-[(4-hydroxyphenyl)methylidene]-2,3-dihydro-1H-indene-4,6-diol |
|---|
| Traditional Name | (1Z)-3-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)-1-[(4-hydroxyphenyl)methylidene]-2,3-dihydroindene-4,6-diol |
|---|
| SMILES | OC1=CC=C(\C=C2\C(C(C3=C2C=C(O)C=C3O)C2=CC(O)=CC(O)=C2)C2=CC=C(O)C=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C28H22O6/c29-18-5-1-15(2-6-18)9-23-24-13-22(33)14-25(34)28(24)27(17-10-20(31)12-21(32)11-17)26(23)16-3-7-19(30)8-4-16/h1-14,26-27,29-34H/b23-9+ |
|---|
| InChI Key | NJFRRNXUFGQUEK-NUGSKGIGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Indane
- Resorcinol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002b-0109700000-7539fc3f07c972d57f84 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0a4i-1000059000-c952f30f264d56d5087e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0102900000-9af9b3b7f04b50729753 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6s-0418900000-879227e0a0e6c19042fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0570-0116900000-3b4dd759bfa3ed4fb4d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-70a8554afb14fc362c14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000900000-3a900bacbd40a7c775d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-0104900000-e44f76836e46b5e5eb8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0001900000-b2735b581da283fe3706 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0008900000-55b0d3399963f5dbee33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-1019200000-54456dd2a36b337bf710 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-f06521fa4057fc1d6ca8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000900000-c77a402dcdfd58c82bd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-0009400000-318d42cb2f385837a349 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040894 |
|---|
| FooDB ID | FDB020730 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00015739 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015043 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752975 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|