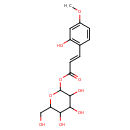| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:35:49 UTC |
|---|
| Update Date | 2016-11-09 01:21:03 UTC |
|---|
| Accession Number | CHEM034009 |
|---|
| Identification |
|---|
| Common Name | 1-O-2'-Hydroxy-4'-methoxycinnamoyl-b-D-glucose |
|---|
| Class | Small Molecule |
|---|
| Description | 1-O-2'-Hydroxy-4'-methoxycinnamoyl-b-D-glucose is found in fats and oils. 1-O-2'-Hydroxy-4'-methoxycinnamoyl-b-D-glucose is a constituent of Matricaria chamomilla (German chamomile). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl (2E)-3-(2-hydroxy-4-methoxyphenyl)prop-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C16H20O9 |
|---|
| Average Molecular Mass | 356.325 g/mol |
|---|
| Monoisotopic Mass | 356.111 g/mol |
|---|
| CAS Registry Number | 145227-80-1 |
|---|
| IUPAC Name | 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl (2E)-3-(2-hydroxy-4-methoxyphenyl)prop-2-enoate |
|---|
| Traditional Name | 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl (2E)-3-(2-hydroxy-4-methoxyphenyl)prop-2-enoate |
|---|
| SMILES | COC1=CC(O)=C(\C=C\C(=O)OC2OC(CO)C(O)C(O)C2O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H20O9/c1-23-9-4-2-8(10(18)6-9)3-5-12(19)25-16-15(22)14(21)13(20)11(7-17)24-16/h2-6,11,13-18,20-22H,7H2,1H3/b5-3+ |
|---|
| InChI Key | VUSUWXXWRAWGIH-HWKANZROSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxycinnamic acid glycosides. These are glycosylated hydoxycinnamic acids derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Hydroxycinnamic acid glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxycinnamic acid glycoside
- O-cinnamoyl glycoside
- Hexose monosaccharide
- Hydroxycinnamic acid
- Cinnamic acid ester
- Methoxyphenol
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Styrene
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Fatty acid ester
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Oxane
- Benzenoid
- Monosaccharide
- Fatty acyl
- Monocyclic benzene moiety
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Secondary alcohol
- Carboxylic acid ester
- Polyol
- Oxacycle
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Carboxylic acid derivative
- Acetal
- Ether
- Primary alcohol
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kii-9723000000-ab0dd943ebf363c0e185 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0059-2273029000-37278f414f96ef31c403 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06tk-0902000000-c516fe75ac5d15858561 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002k-1900000000-bd6a50a0d9b904f188c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-4900000000-26657609b9c041eb8e4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0902000000-339aaee0cfdd4abb8a57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01tc-2900000000-94edb82292329dba706a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5900000000-145ed16775c05f9c7f72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-969167d17423b510e29d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-1900000000-cf7bb93dde67aad26f3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0535-4901000000-9d2c913c9e2a2e0f6a50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0bvj-0906000000-83019ded77c4cab2c599 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-6f1c860dc30cad2d08c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059m-3921000000-87519be2363bde12d640 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040866 |
|---|
| FooDB ID | FDB020695 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 168915 |
|---|
| PubChem Compound ID | 131752961 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|