| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:33:39 UTC |
|---|
| Update Date | 2016-11-09 01:21:02 UTC |
|---|
| Accession Number | CHEM033965 |
|---|
| Identification |
|---|
| Common Name | (S)-11,12,13-Trinor-7-calamenone |
|---|
| Class | Small Molecule |
|---|
| Description | xi-11,12,13-Trinor-7-calamenone is found in root vegetables. xi-11,12,13-Trinor-7-calamenone is a constituent of Cyperus rotundus (nutgrass). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
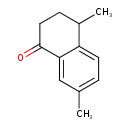
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C12H14O |
|---|
| Average Molecular Mass | 174.239 g/mol |
|---|
| Monoisotopic Mass | 174.104 g/mol |
|---|
| CAS Registry Number | 155748-76-8 |
|---|
| IUPAC Name | 4,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-one |
|---|
| Traditional Name | 4,7-dimethyl-3,4-dihydro-2H-naphthalen-1-one |
|---|
| SMILES | CC1CCC(=O)C2=CC(C)=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C12H14O/c1-8-3-5-10-9(2)4-6-12(13)11(10)7-8/h3,5,7,9H,4,6H2,1-2H3 |
|---|
| InChI Key | SQESYXTWWGWCFK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetralins. These are polycyclic aromatic compounds containing a tetralin moiety, which consists of a benzene fused to a cyclohexane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Tetralins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetralins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetralin
- Aryl alkyl ketone
- Aryl ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052b-1900000000-068ed35355ad6f8eb8b7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-845b3d511a88cd25bd00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05r0-2900000000-3f5c5c8954fee3dbf592 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9800000000-37ec55543652ccb52b6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-76a1ae831f45f91da470 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-c41e69258aab2f209d65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-3900000000-bd2dfebcbc04d686f5b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-7e348a3518409b2410fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-a2818572ee4035836604 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5900000000-a7d96684e696bb08a32c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-92c23d6919131ddb4b3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1900000000-09aca0af15a5f4c94445 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9800000000-799b63887f186c12fc55 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040823 |
|---|
| FooDB ID | FDB021512 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4475869 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5316902 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|