| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:32:41 UTC |
|---|
| Update Date | 2016-11-09 01:21:02 UTC |
|---|
| Accession Number | CHEM033941 |
|---|
| Identification |
|---|
| Common Name | 11-Hydroxytubotaiwine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from cultured cells of Aspidosperma quebracho-blanco (quebracho) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
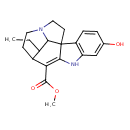
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 18-ethyl-5-hydroxy-8,14-diazapentacyclo[9.5.2.0¹,⁹.0²,⁷.0¹⁴,¹⁷]octadeca-2(7),3,5,9-tetraene-10-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C20H24N2O3 |
|---|
| Average Molecular Mass | 340.416 g/mol |
|---|
| Monoisotopic Mass | 340.179 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | methyl 18-ethyl-5-hydroxy-8,14-diazapentacyclo[9.5.2.0¹,⁹.0²,⁷.0¹⁴,¹⁷]octadeca-2(7),3,5,9-tetraene-10-carboxylate |
|---|
| Traditional Name | methyl 18-ethyl-5-hydroxy-8,14-diazapentacyclo[9.5.2.0¹,⁹.0²,⁷.0¹⁴,¹⁷]octadeca-2(7),3,5,9-tetraene-10-carboxylate |
|---|
| SMILES | CCC1C2N3CCC22C(NC4=C2C=CC(O)=C4)=C(C1CC3)C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C20H24N2O3/c1-3-12-13-6-8-22-9-7-20(18(12)22)14-5-4-11(23)10-15(14)21-17(20)16(13)19(24)25-2/h4-5,10,12-13,18,21,23H,3,6-9H2,1-2H3 |
|---|
| InChI Key | ILEBWSZOKJHVSX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as strychnos alkaloids. These are alkaloids having a core structure based on the strychnan, stemmadenine (seco-curan), or the akuammicine (curan) skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Strychnos alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Strychnos alkaloids |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0400-2169000000-426dc92a36a4349a0442 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-001j-1019000000-a0cb0c0ddbd64676da93 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-6b10e1a580d1efdba6d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0536-0039000000-ad8097ec6e6d6226d076 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gir-0290000000-4da506b083a0d6174149 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0019000000-c20cbe31aa7e393ce65d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-0059000000-791c85e68729835da8be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ue9-0090000000-5b140cf38f837962a67b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052r-0009000000-437d0197c047eb2cc720 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-035d42d01b5221877b99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0059000000-72216c1022f0fe4e98a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-3b43e0643b1819424eeb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0019000000-2ba77f2622a02ac512a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00m0-0095000000-01a6f2f8eecca4c6877a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040799 |
|---|
| FooDB ID | FDB020615 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752942 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|