| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:32:39 UTC |
|---|
| Update Date | 2016-11-09 01:21:02 UTC |
|---|
| Accession Number | CHEM033940 |
|---|
| Identification |
|---|
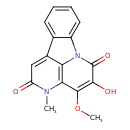
| Common Name | 5-Hydroxy-4-methoxy-3-methyl-2,6-canthinedione |
|---|
| Class | Small Molecule |
|---|
| Description | 5-Hydroxy-4-methoxy-3-methyl-2,6-canthinedione is an alkaloid from the wood of Quassia amara (Surinam quassia). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Ethoxy-5-(1-propenyl)phenol, 9ci | HMDB | | 2-Ethoxy-5-[(1E)-1-propenyl]phenol | HMDB | | 5-Propenylguaethol | HMDB | | 6-Ethoxy-m-anol | HMDB | | FEMA 2922 | HMDB | | Hydroxy methyl anethol | HMDB | | Isosafroeugenol | HMDB | | Isosafroeugenole | HMDB | | Propenyl guaethol | HMDB | | Propenylguaethol | HMDB | | Propenylguaethole | HMDB | | Propenylguaetol | HMDB | | trans-2-Ethoxy-5-(1-propenyl)phenol | HMDB | | Vanitrope | HMDB |
|
|---|
| Chemical Formula | C16H12N2O4 |
|---|
| Average Molecular Mass | 296.278 g/mol |
|---|
| Monoisotopic Mass | 296.080 g/mol |
|---|
| CAS Registry Number | 129724-30-7 |
|---|
| IUPAC Name | 3-hydroxy-4-methoxy-6-methyl-1,6-diazatetracyclo[7.6.1.0⁵,¹⁶.0¹⁰,¹⁵]hexadeca-3,5(16),8,10,12,14-hexaene-2,7-dione |
|---|
| Traditional Name | 3-hydroxy-4-methoxy-6-methyl-1,6-diazatetracyclo[7.6.1.0⁵,¹⁶.0¹⁰,¹⁵]hexadeca-3,5(16),8,10,12,14-hexaene-2,7-dione |
|---|
| SMILES | COC1=C(O)C(=O)N2C3=CC=CC=C3C3=CC(=O)N(C)C1=C23 |
|---|
| InChI Identifier | InChI=1S/C16H12N2O4/c1-17-11(19)7-9-8-5-3-4-6-10(8)18-12(9)13(17)15(22-2)14(20)16(18)21/h3-7,20H,1-2H3 |
|---|
| InChI Key | CQFOPDQAOBCODL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolonaphthyridine alkaloids. These are a numerous and relatively straightforward subgroup of the b-carbolines, e.g. Canthin-6-one, in which an additional C3 unit is attached between C-1 and the indole nitrogen to form an additional ring. The group includes a few dimeric examples, such as Haplophytine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Indolonaphthyridine alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Indolonaphthyridine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolo[3,2-1de][1,5]naphthyridine
- Pyridoindolone
- Beta-carboline
- Pyridoindole
- Diazanaphthalene
- Naphthyridine
- Indole
- Indole or derivatives
- Indolizine
- Pyrrolopyridine
- Alkyl aryl ether
- Hydroxypyridine
- Pyridinone
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Vinylogous ester
- Pyrrole
- Lactam
- Azacycle
- Ether
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-0090000000-f18fcc70ae2cd3888af2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0uka-2139000000-47259223f378ce840a1a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-03d0a78eadc85e3d2ec2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0090000000-e496b4287fadced66a79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-0940000000-77d22c5f183d7770885e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-b62122769f7eda50543e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-a9992798466a69d73901 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dr-1590000000-3c1312713ef14deab011 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-8c0a37cb17704175d425 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-8c0a37cb17704175d425 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1290000000-1772faac4465b5d9c0e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-5c316f709f7b20344764 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-5c316f709f7b20344764 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0950000000-4a637b057ae6fdd47a1f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040798 |
|---|
| FooDB ID | FDB020614 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777512 |
|---|
| ChEBI ID | 174129 |
|---|
| PubChem Compound ID | 5354280 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|