| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:32:11 UTC |
|---|
| Update Date | 2016-11-09 01:21:02 UTC |
|---|
| Accession Number | CHEM033926 |
|---|
| Identification |
|---|
| Common Name | (3beta,19alpha)-3,19,23,24-Tetrahydroxy-12-oleanen-28-oic acid |
|---|
| Class | Small Molecule |
|---|
| Description | (3beta,19alpha)-3,19,23,24-Tetrahydroxy-12-oleanen-28-oic acid is found in fruits. (3beta,19alpha)-3,19,23,24-Tetrahydroxy-12-oleanen-28-oic acid is a constituent of the wild guava Alibertia edulis. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
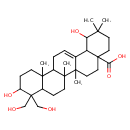
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3b,19a)-3,19,23,24-Tetrahydroxy-12-oleanen-28-Oate | Generator | | (3b,19a)-3,19,23,24-Tetrahydroxy-12-oleanen-28-Oic acid | Generator | | (3beta,19alpha)-3,19,23,24-Tetrahydroxy-12-oleanen-28-Oate | Generator | | (3Β,19α)-3,19,23,24-tetrahydroxy-12-oleanen-28-Oate | Generator | | (3Β,19α)-3,19,23,24-tetrahydroxy-12-oleanen-28-Oic acid | Generator | | 1,10-Dihydroxy-9,9-bis(hydroxymethyl)-2,2,6a,6b,12a-pentamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate | HMDB |
|
|---|
| Chemical Formula | C30H48O6 |
|---|
| Average Molecular Mass | 504.699 g/mol |
|---|
| Monoisotopic Mass | 504.345 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 1,10-dihydroxy-9,9-bis(hydroxymethyl)-2,2,6a,6b,12a-pentamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid |
|---|
| Traditional Name | 1,10-dihydroxy-9,9-bis(hydroxymethyl)-2,2,6a,6b,12a-pentamethyl-1,3,4,5,6,7,8,8a,10,11,12,12b,13,14b-tetradecahydropicene-4a-carboxylic acid |
|---|
| SMILES | CC1(C)CCC2(CCC3(C)C(=CCC4C5(C)CCC(O)C(CO)(CO)C5CCC34C)C2C1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C30H48O6/c1-25(2)12-14-29(24(35)36)15-13-27(4)18(22(29)23(25)34)6-7-19-26(3)10-9-21(33)30(16-31,17-32)20(26)8-11-28(19,27)5/h6,19-23,31-34H,7-17H2,1-5H3,(H,35,36) |
|---|
| InChI Key | SWKIMTSJFCDAEL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Steroid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002r-1007900000-a2c3d056b1023402cbde | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-1003019000-b5a238da751f1551f000 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0000910000-e857a10c7a0b42c4b1d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0001900000-91cc8b69441c6ff5fe31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-2115900000-f8d4db596574c86d9f3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udr-0000960000-997e83c87d3dceabc54b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-0000910000-ad6a839a55d7d84c43de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-1000900000-7dfadf52b36f1c4f8389 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000090000-0ac24b9167c1f1bdf7db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05i0-0000900000-c904711d308886db5574 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0000900000-6b4bebeced1609c74b12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000690000-799baad269f1a4ecd44b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4u-0101910000-8eeb77603d7414c27b96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-3907400000-8b407bde1f935eb6c2c9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040784 |
|---|
| FooDB ID | FDB020600 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015026 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752936 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|