| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:30:15 UTC |
|---|
| Update Date | 2016-11-09 01:21:01 UTC |
|---|
| Accession Number | CHEM033881 |
|---|
| Identification |
|---|
| Common Name | Haematopodin |
|---|
| Class | Small Molecule |
|---|
| Description | Pigment from fruiting bodies of the toadstool Mycena haematopus (blood mycena). Haematopodin is found in root vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
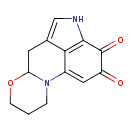
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C13H12N2O3 |
|---|
| Average Molecular Mass | 244.246 g/mol |
|---|
| Monoisotopic Mass | 244.085 g/mol |
|---|
| CAS Registry Number | 151964-21-5 |
|---|
| IUPAC Name | 6-oxa-2,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(15),9,12(16)-triene-13,14-dione |
|---|
| Traditional Name | 6-oxa-2,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(15),9,12(16)-triene-13,14-dione |
|---|
| SMILES | O=C1C=C2N3CCCOC3CC3=CNC(=C23)C1=O |
|---|
| InChI Identifier | InChI=1S/C13H12N2O3/c16-9-5-8-11-7(6-14-12(11)13(9)17)4-10-15(8)2-1-3-18-10/h5-6,10,14H,1-4H2 |
|---|
| InChI Key | GTVMGUBGOSWMOJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolo[4,3,2-de]quinolines. These are organic heterocyclic compounds with a structure based on the Pyrrolo[4,3,2-de]quinoline skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Pyrroloquinolines |
|---|
| Direct Parent | Pyrrolo[4,3,2-de]quinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolo[4,3,2-de]quinoline
- Indole or derivatives
- Aryl ketone
- 1,3-oxazinane
- Oxazinane
- Pyrrole
- Heteroaromatic compound
- Vinylogous amide
- Ketone
- Cyclic ketone
- Enamine
- Oxacycle
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-1890000000-ea9bd2586c4ab600beac | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-cafbfd9f95ea05094175 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-1090000000-4e5d3a811cff891bdcba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9800000000-13a576e6bf41b3a45077 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-692665977925d05a1d98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f7c-1890000000-beffa2b8403a2524f852 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054x-5900000000-f4dff54182b297a96dea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-215c5b93a9265e717362 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-215c5b93a9265e717362 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-0980000000-70a15129d6f8c723cbb1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-dfaae9315458788603d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0190000000-c4bc645613a1aa8d33fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0960000000-418226193c3ff5f7e801 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040734 |
|---|
| FooDB ID | FDB020544 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Haematopodin |
|---|
| Chemspider ID | 9033000 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10857709 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|