| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:28:32 UTC |
|---|
| Update Date | 2016-11-09 01:21:01 UTC |
|---|
| Accession Number | CHEM033843 |
|---|
| Identification |
|---|
| Common Name | 4R,5R,6S-Trihydroxy-2-hydroxymethyl-2-cyclohexen-1-one 6-(2-hydroxy-6-methylbenzoate) |
|---|
| Class | Small Molecule |
|---|
| Description | 4R,5R,6S-Trihydroxy-2-hydroxymethyl-2-cyclohexen-1-one 6-(2-hydroxy-6-methylbenzoate) is found in green vegetables. 4R,5R,6S-Trihydroxy-2-hydroxymethyl-2-cyclohexen-1-one 6-(2-hydroxy-6-methylbenzoate) is produced by a Phoma sp. on leaves and stalks of rhubarb. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
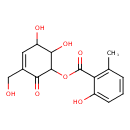
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4R,5R,6S-Trihydroxy-2-hydroxymethyl-2-cyclohexen-1-one 6-(2-hydroxy-6-methylbenzoic acid) | Generator | | 5,6-Dihydroxy-3-(hydroxymethyl)-2-oxocyclohex-3-en-1-yl 2-hydroxy-6-methylbenzoic acid | Generator |
|
|---|
| Chemical Formula | C15H16O7 |
|---|
| Average Molecular Mass | 308.283 g/mol |
|---|
| Monoisotopic Mass | 308.090 g/mol |
|---|
| CAS Registry Number | 146475-71-0 |
|---|
| IUPAC Name | 5,6-dihydroxy-3-(hydroxymethyl)-2-oxocyclohex-3-en-1-yl 2-hydroxy-6-methylbenzoate |
|---|
| Traditional Name | 5,6-dihydroxy-3-(hydroxymethyl)-2-oxocyclohex-3-en-1-yl 2-hydroxy-6-methylbenzoate |
|---|
| SMILES | CC1=C(C(=O)OC2C(O)C(O)C=C(CO)C2=O)C(O)=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C15H16O7/c1-7-3-2-4-9(17)11(7)15(21)22-14-12(19)8(6-16)5-10(18)13(14)20/h2-5,10,13-14,16-18,20H,6H2,1H3 |
|---|
| InChI Key | ZJIDZZXKQOJXMR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-hydroxybenzoic acid esters. These are benzoic acid esters where the benzene ring is ortho-substituted with a hydroxy group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | o-Hydroxybenzoic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-hydroxybenzoic acid ester
- Salicylic acid or derivatives
- Benzoyl
- M-cresol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Cyclohexenone
- Phenol
- Toluene
- Cyclitol or derivatives
- Vinylogous acid
- Ketone
- Cyclic ketone
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-9410000000-758cc2b54f6bf18d8580 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-053r-2293080000-90c6536a7d067d5cc9da | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0987000000-18a2dcb963cadc098d53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052u-0961000000-5f295d3f12b7504f3b0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-9800000000-48ed3d30485c0b5c6859 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0549000000-2f953dbc68471c0dc810 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0931000000-bb7923398079220ceedb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1900000000-f6535998b006e25cf2ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0394000000-b3722ed09feb088178e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-1920000000-11871629e30545587856 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0673-6900000000-2aee85abc1a539db50db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pc9-0922000000-e03a598318cbd4adab4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0910000000-968206ea7a784c988636 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-3900000000-f18b484958dd2964f12e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040692 |
|---|
| FooDB ID | FDB020494 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 20057935 |
|---|
| ChEBI ID | 174940 |
|---|
| PubChem Compound ID | 22297980 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|