| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:28:13 UTC |
|---|
| Update Date | 2016-11-09 01:21:00 UTC |
|---|
| Accession Number | CHEM033839 |
|---|
| Identification |
|---|
| Common Name | (3x,5x,10x)-9,10-Didehydroisohumbertiol O-[rhamnosyl-(1->4)-rhamnosyl-(1->2)-[rhamnosyl-(1->6)]-glucoside] |
|---|
| Class | Small Molecule |
|---|
| Description | (3x,5x,10x)-9,10-Didehydroisohumbertiol O-[rhamnosyl-(1->4)-rhamnosyl-(1->2)-[rhamnosyl-(1->6)]-glucoside] is found in fruits. (3x,5x,10x)-9,10-Didehydroisohumbertiol O-[rhamnosyl-(1->4)-rhamnosyl-(1->2)-[rhamnosyl-(1->6)]-glucoside] is a constituent of Eriobotrya japonica (loquat). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
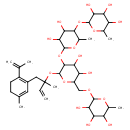
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C39H62O18 |
|---|
| Average Molecular Mass | 818.899 g/mol |
|---|
| Monoisotopic Mass | 818.394 g/mol |
|---|
| CAS Registry Number | 143376-49-2 |
|---|
| IUPAC Name | 2-{[5-({3,4-dihydroxy-6-methyl-5-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl}oxy)-3,4-dihydroxy-6-({2-methyl-1-[5-methyl-2-(prop-1-en-2-yl)cyclohexa-1,5-dien-1-yl]but-3-en-2-yl}oxy)oxan-2-yl]methoxy}-6-methyloxane-3,4,5-triol |
|---|
| Traditional Name | 2-{[5-({3,4-dihydroxy-6-methyl-5-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl}oxy)-3,4-dihydroxy-6-({2-methyl-1-[5-methyl-2-(prop-1-en-2-yl)cyclohexa-1,5-dien-1-yl]but-3-en-2-yl}oxy)oxan-2-yl]methoxy}-6-methyloxane-3,4,5-triol |
|---|
| SMILES | CC1OC(OCC2OC(OC(C)(CC3=C(CCC(C)=C3)C(C)=C)C=C)C(OC3OC(C)C(OC4OC(C)C(O)C(O)C4O)C(O)C3O)C(O)C2O)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C39H62O18/c1-9-39(8,13-20-12-16(4)10-11-21(20)15(2)3)57-38-34(28(45)25(42)22(54-38)14-50-35-30(47)26(43)23(40)17(5)51-35)56-37-32(49)29(46)33(19(7)53-37)55-36-31(48)27(44)24(41)18(6)52-36/h9,12,17-19,22-38,40-49H,1-2,10-11,13-14H2,3-8H3 |
|---|
| InChI Key | BFNFFYBOJHJTBC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- Cyclofarsesane sesquiterpenoid
- Sesquiterpenoid
- O-glycosyl compound
- Glycosyl compound
- Oxane
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ldi-0291065040-fe1562915f4974b0edcf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-0492052000-4c13f11f38aeff160baa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gba-0982051010-79b14135294b8801fa36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-3792274440-d312739d72f008c84223 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066s-5983063200-e8f1574956dc6959d28a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02t9-2791120000-98f7e33e617f21dd1a83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0910001020-f451f8815fa57cd66e4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-3910000000-ed368caed08194e87879 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015c-8940120010-6236f6e44d6db790d3f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0400013890-e0fadd95203c3a1f32a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-7500015920-47f7933f94ecff63ffd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-8902064310-f52475d5bf3468cecb49 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040687 |
|---|
| FooDB ID | FDB020486 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 168618 |
|---|
| PubChem Compound ID | 131752907 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|