| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:22:47 UTC |
|---|
| Update Date | 2016-11-09 01:20:59 UTC |
|---|
| Accession Number | CHEM033727 |
|---|
| Identification |
|---|
| Common Name | Adlumidiceine |
|---|
| Class | Small Molecule |
|---|
| Description | Adlumidiceine is an alkaloid from Papaver rhoeas (corn poppy). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
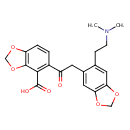
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-[[6-[2-(dimethylamino)Ethyl]-1,3-benzodioxol-5-yl]acetyl]-1,3-benzodioxole-4-carboxylic acid, 9ci | HMDB | | 5-(2-{6-[2-(dimethylamino)ethyl]-2H-1,3-benzodioxol-5-yl}acetyl)-2H-1,3-benzodioxole-4-carboxylate | Generator |
|
|---|
| Chemical Formula | C21H21NO7 |
|---|
| Average Molecular Mass | 399.394 g/mol |
|---|
| Monoisotopic Mass | 399.132 g/mol |
|---|
| CAS Registry Number | 51059-65-5 |
|---|
| IUPAC Name | 5-(2-{6-[2-(dimethylamino)ethyl]-2H-1,3-benzodioxol-5-yl}acetyl)-2H-1,3-benzodioxole-4-carboxylic acid |
|---|
| Traditional Name | 5-(2-{6-[2-(dimethylamino)ethyl]-2H-1,3-benzodioxol-5-yl}acetyl)-2H-1,3-benzodioxole-4-carboxylic acid |
|---|
| SMILES | CN(C)CCC1=CC2=C(OCO2)C=C1CC(=O)C1=C(C(O)=O)C2=C(OCO2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C21H21NO7/c1-22(2)6-5-12-8-17-18(28-10-27-17)9-13(12)7-15(23)14-3-4-16-20(29-11-26-16)19(14)21(24)25/h3-4,8-9H,5-7,10-11H2,1-2H3,(H,24,25) |
|---|
| InChI Key | ZWRGIDKDYJXLHP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Benzodioxole
- Phenethylamine
- Aryl ketone
- Aryl alkyl ketone
- Aralkylamine
- Benzenoid
- Amino acid or derivatives
- Ketone
- Tertiary amine
- Tertiary aliphatic amine
- Amino acid
- Acetal
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-9814000000-bbe3dbbb57aa125870c5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0aor-9081400000-97a55f4e45d6c8607b11 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0109300000-1d991c45ec584e2e0ef2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-1917000000-d70d2ce3801b285396e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-0901000000-0459ad44bb162c4ee6e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0009000000-7ec7b5f0ba44f55f18c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0109000000-87bc3b5e28063d85fb74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-075j-2449000000-447f37c315814e4157cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0k92-0009000000-f3088e7b8f04c6593145 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-a5e85de0d329b6b4c9a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-2529000000-07f124bc406639f8f0dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009800000-4d33e5c9630dafbce47b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0109000000-76391d0da443775eec51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-1923000000-8758c9604ab41474c536 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040552 |
|---|
| FooDB ID | FDB020327 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027269 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777492 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12866383 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|