| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:22:10 UTC |
|---|
| Update Date | 2016-11-09 01:20:59 UTC |
|---|
| Accession Number | CHEM033711 |
|---|
| Identification |
|---|
| Common Name | Artomunoxanthentrione epoxide |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from the root bark of Artocarpus communis (breadfruit). Artomunoxanthentrione epoxide is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
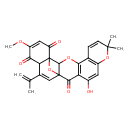
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C26H22O8 |
|---|
| Average Molecular Mass | 462.448 g/mol |
|---|
| Monoisotopic Mass | 462.131 g/mol |
|---|
| CAS Registry Number | 143522-33-2 |
|---|
| IUPAC Name | 12-hydroxy-20-methoxy-8,8-dimethyl-17-(prop-1-en-2-yl)-3,9,23-trioxahexacyclo[13.7.1.0¹,¹⁸.0²,¹⁵.0⁴,¹³.0⁵,¹⁰]tricosa-4(13),5(10),6,11,16,20-hexaene-14,19,22-trione |
|---|
| Traditional Name | 12-hydroxy-20-methoxy-8,8-dimethyl-17-(prop-1-en-2-yl)-3,9,23-trioxahexacyclo[13.7.1.0¹,¹⁸.0²,¹⁵.0⁴,¹³.0⁵,¹⁰]tricosa-4(13),5(10),6,11,16,20-hexaene-14,19,22-trione |
|---|
| SMILES | COC1=CC(=O)C23OC4(C=C(C2C1=O)C(C)=C)C3OC1=C(C(O)=CC2=C1C=CC(C)(C)O2)C4=O |
|---|
| InChI Identifier | InChI=1S/C26H22O8/c1-11(2)13-10-25-22(30)18-14(27)8-15-12(6-7-24(3,4)33-15)21(18)32-23(25)26(34-25)17(28)9-16(31-5)20(29)19(13)26/h6-10,19,23,27H,1H2,2-5H3 |
|---|
| InChI Key | NDLOJOBMSNOREU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranoxanthones. These are organic aromatic compounds containing a pyran or a hydrogenated derivative fused to a xanthone ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Pyranoxanthones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyranoxanthone
- Naphthopyran
- Pyranochromene
- 2,2-dimethyl-1-benzopyran
- Chromone
- Naphthalene
- Aryl alkyl ketone
- Aryl ketone
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Cyclohexenone
- Pyran
- Benzenoid
- Vinylogous ester
- Vinylogous acid
- Oxetane
- Ketone
- Oxacycle
- Ether
- Dialkyl ether
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01qd-7301900000-165ae9f61b9b20029be3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0aor-9330260000-21f600558731b5655e7d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-135522ccc3af3d788697 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0301-2061900000-16ccd0301666c1e7a6f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-5940000000-8df62234e783b8adbbf5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0010900000-aff4b3d70741e2832fbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1011900000-f2ea2049b93995a8bf8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aru-6982000000-8a748a350724c38b44cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-9208e3ee3180b8cb41df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000900000-4df73206b8b8faa7de44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03k9-1001900000-cbea8bae195f2e91da52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-9a0269f6702280e74668 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000900000-9e56d292e999e4a8eb14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1012900000-0e2dbe2dc18ec4f37098 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040533 |
|---|
| FooDB ID | FDB020301 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752846 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|