| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:22:06 UTC |
|---|
| Update Date | 2016-11-09 01:20:59 UTC |
|---|
| Accession Number | CHEM033709 |
|---|
| Identification |
|---|
| Common Name | 2-Hydroxy-4-oxopentanoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Hydroxy-4-oxopentanoic acid is found in alcoholic beverages. 2-Hydroxy-4-oxopentanoic acid is formed in beer wort fermentation Alitretinoin (9-cis-retinoic acid) is a naturally-occurring endogenous retinoid indicated for topical treatment of cutaneous lesions in patients with AIDS-related Kaposi's sarcoma. Alitretinoin inhibits the growth of Kaposi's sarcoma (KS) cells in vitro. Retinoic acid is the oxidized form of Vitamin A. It functions in determining position along embryonic anterior/posterior axis in chordates. It acts through Hox genes, which ultimately control anterior/posterior patterning in early developmental stages. Retinoic acid acts by binding to heterodimers of the retinoic acid receptor (RAR) and the retinoid X receptor (RXR), which then bind to retinoic acid response elements (RAREs) in the regulatory regions of direct targets (including Hox genes), thereby activating gene transcription. Retinoic acid receptors mediate transcription of different sets of genes of cell differentiation, thus it also depends on the target cells. 2-Hydroxy-4-oxopentanoic acid is one of the target genes is the gene of the retinoic acid receptor itself which occurs during positive regulation. Control of retinoic acid levels is maintained by a suite of proteins. Retinoic acid is the oxidized form of Vitamin A. It functions in determining position along embryonic anterior/posterior axis in chordates. It acts through Hox genes, which ultimately controls anterior/posterior patterning in early developmental stages (PMID: 17495912). It is an important regulator of gene expression during growth and development, and in neoplasms. Tretinoin, also known as retinoic acid and derived from maternal vitamin A, is essential for normal growth and embryonic development. 2-Hydroxy-4-oxopentanoic acid is an excess of tretinoin can be teratogenic. It is used in the treatment of psoriasis; acne vulgaris; and several other skin diseases. It has also been approved for use in promyelocytic leukemia (leukemia, promyelocytic, acute). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
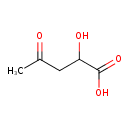
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxy-4-oxopentanoate | Generator | | 2-Hydroxy-4-oxovaleric acid | HMDB | | a-Hydroxy-b-acetylpropionic acid | HMDB | | a-Hydroxylevulinic acid | HMDB | | Acetolactic acid | HMDB |
|
|---|
| Chemical Formula | C5H8O4 |
|---|
| Average Molecular Mass | 132.115 g/mol |
|---|
| Monoisotopic Mass | 132.042 g/mol |
|---|
| CAS Registry Number | 54031-97-9 |
|---|
| IUPAC Name | 2-hydroxy-4-oxopentanoic acid |
|---|
| Traditional Name | 2-hydroxy-4-oxopentanoic acid |
|---|
| SMILES | CC(=O)CC(O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H8O4/c1-3(6)2-4(7)5(8)9/h4,7H,2H2,1H3,(H,8,9) |
|---|
| InChI Key | QTSNVMMGKAPSRT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma-keto acids and derivatives. These are organic compounds containing an aldehyde substituted with a keto group on the C4 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Gamma-keto acids and derivatives |
|---|
| Direct Parent | Gamma-keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma-keto acid
- Short-chain keto acid
- Alpha-hydroxy acid
- Beta-hydroxy ketone
- Hydroxy acid
- Secondary alcohol
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-e66d04210bcdaaec0f1f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0076-9340000000-7e77e0de3e7fb5124421 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-7900000000-134b9829ef446615f81e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-9300000000-8172fac34150117643bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-1ba9dbbb6e61f679b4c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-6900000000-18c236e3427ea498fa32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01w0-9300000000-431e5906f2009cacfd36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0avu-9000000000-12a74b10e2c2bfca1fb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01q9-7900000000-c98cddec2196e78ceb4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-7df4f2d0321e470c3da6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-3f203d80defce9fc9425 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-9000000000-8ebf358e48c5c00cee6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-d5b8515c60ed019f79b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-3a78789c386089a42b49 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040531 |
|---|
| FooDB ID | FDB020299 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8278736 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10103208 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|