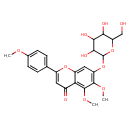| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:21:26 UTC |
|---|
| Update Date | 2016-11-09 01:20:59 UTC |
|---|
| Accession Number | CHEM033692 |
|---|
| Identification |
|---|
| Common Name | 4',5,6-Trimethylscutellarein 7-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | 4',5,6-Trimethylscutellarein 7-glucoside is found in fruits. 4',5,6-Trimethylscutellarein 7-glucoside is a constituent of Juniperus communis (juniper). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C24H26O11 |
|---|
| Average Molecular Mass | 490.457 g/mol |
|---|
| Monoisotopic Mass | 490.148 g/mol |
|---|
| CAS Registry Number | 17680-85-2 |
|---|
| IUPAC Name | 5,6-dimethoxy-2-(4-methoxyphenyl)-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-chromen-4-one |
|---|
| Traditional Name | 5,6-dimethoxy-2-(4-methoxyphenyl)-7-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}chromen-4-one |
|---|
| SMILES | COC1=CC=C(C=C1)C1=CC(=O)C2=C(OC)C(OC)=C(OC3OC(CO)C(O)C(O)C3O)C=C2O1 |
|---|
| InChI Identifier | InChI=1S/C24H26O11/c1-30-12-6-4-11(5-7-12)14-8-13(26)18-15(33-14)9-16(22(31-2)23(18)32-3)34-24-21(29)20(28)19(27)17(10-25)35-24/h4-9,17,19-21,24-25,27-29H,10H2,1-3H3 |
|---|
| InChI Key | GCAYMASOWMSYII-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthraquinones. These are organic compounds containing either anthracene-9,10-quinone, 1,4-anthraquinone, or 1,2-anthraquinone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthraquinones |
|---|
| Direct Parent | Anthraquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 9,10-anthraquinone
- Anthraquinone
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Anisole
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Oxane
- Monosaccharide
- Vinylogous acid
- Secondary alcohol
- Ketone
- Acetal
- Oxacycle
- Ether
- Organoheterocyclic compound
- Polyol
- Alcohol
- Organic oxygen compound
- Organic oxide
- Primary alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-9303700000-f25718d4f0c9bd115b8a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-06fr-5443039000-7aed76a02841c8ecc2f4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0203-0009700000-f4ddfab38a22a881905c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-0109000000-c437535a632e0f070669 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-2469000000-364022ff551a678fa301 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-1205900000-bad9e951c87b6bd792d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-1109300000-df31ddff0b33e11b1913 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fr-3249000000-f6f874bdabe06b950efe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-17ff811044a5a7c5a88e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0000900000-82bd97dfbca5a770a7c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p9-0041900000-e1642d3ecb8f641aba38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000900000-518f99accdb752528084 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000900000-ba421753c8be881dc846 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0001900000-7fa2ddc8df0b365f8561 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040512 |
|---|
| FooDB ID | FDB020278 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 169832 |
|---|
| PubChem Compound ID | 131752841 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|