| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:19:59 UTC |
|---|
| Update Date | 2016-11-09 01:20:58 UTC |
|---|
| Accession Number | CHEM033657 |
|---|
| Identification |
|---|
| Common Name | 3',7-Dimethoxy-4',5,8-trihydroxyflavone 8-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | 3',7-Dimethoxy-4',5,8-trihydroxyflavone 8-glucoside is found in green vegetables. 3',7-Dimethoxy-4',5,8-trihydroxyflavone 8-glucoside is a constituent of sugar cane syrup. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
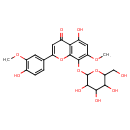
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C23H24O12 |
|---|
| Average Molecular Mass | 492.430 g/mol |
|---|
| Monoisotopic Mass | 492.127 g/mol |
|---|
| CAS Registry Number | 100997-33-9 |
|---|
| IUPAC Name | 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-8-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-chromen-4-one |
|---|
| Traditional Name | 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-8-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}chromen-4-one |
|---|
| SMILES | COC1=C(O)C=CC(=C1)C1=CC(=O)C2=C(O1)C(OC1OC(CO)C(O)C(O)C1O)=C(OC)C=C2O |
|---|
| InChI Identifier | InChI=1S/C23H24O12/c1-31-14-5-9(3-4-10(14)25)13-6-11(26)17-12(27)7-15(32-2)21(22(17)33-13)35-23-20(30)19(29)18(28)16(8-24)34-23/h3-7,16,18-20,23-25,27-30H,8H2,1-2H3 |
|---|
| InChI Key | HAANPBPCWSXUMK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-8-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C8-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-8-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavonoid-8-o-glycoside
- 3p-methoxyflavonoid-skeleton
- 7-methoxyflavonoid-skeleton
- Hydroxyflavonoid
- Flavone
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- Phenolic glycoside
- Hexose monosaccharide
- Chromone
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Pyranone
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Pyran
- Oxane
- Monosaccharide
- Vinylogous acid
- Heteroaromatic compound
- Secondary alcohol
- Ether
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Acetal
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Alcohol
- Primary alcohol
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-074i-9302800000-322197587bf64b84e511 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-7400029000-3d0459c11fc148dff9bb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002f-0205900000-9d81b2ebb65beb488b59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-1319300000-90ffdaf7f977f71b549b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-4469000000-85aa7ba251c37003c2ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-e4e89d9a48bd24d8e837 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002g-0000900000-80793eac61b694911938 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-003u-0009700000-5dfb9bea90182eb29f22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0109100000-4406b052825892901df9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ha-1938000000-611a7dfc309fe787b1db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000900000-fadce352490b60d5c39e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000900000-e7665f3a9f15eeb82a93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0umi-0330900000-a5b543d6b173b535d3c4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040479 |
|---|
| FooDB ID | FDB020238 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752831 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|