| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:15:15 UTC |
|---|
| Update Date | 2016-11-09 01:20:57 UTC |
|---|
| Accession Number | CHEM033551 |
|---|
| Identification |
|---|
| Common Name | Dihydro-4,6-dimethyl-2-(1-methylpropyl)-4H-1,3,5-dithiazine |
|---|
| Class | Small Molecule |
|---|
| Description | Dihydro-4,6-dimethyl-2-(1-methylpropyl)-4H-1,3,5-dithiazine is found in mollusks. Dihydro-4,6-dimethyl-2-(1-methylpropyl)-4H-1,3,5-dithiazine is isolated from dried squid aroma. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
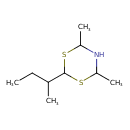
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C9H19NS2 |
|---|
| Average Molecular Mass | 205.384 g/mol |
|---|
| Monoisotopic Mass | 205.096 g/mol |
|---|
| CAS Registry Number | 104691-36-3 |
|---|
| IUPAC Name | 2-(butan-2-yl)-4,6-dimethyl-1,3,5-dithiazinane |
|---|
| Traditional Name | 4,6-dimethyl-2-(sec-butyl)-1,3,5-dithiazinane |
|---|
| SMILES | CCC(C)C1SC(C)NC(C)S1 |
|---|
| InChI Identifier | InChI=1S/C9H19NS2/c1-5-6(2)9-11-7(3)10-8(4)12-9/h6-10H,5H2,1-4H3 |
|---|
| InChI Key | BLDFHTPIEUHZJY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3,5-dithiazinanes. These are cyclic compounds that contain a dithiazinane ring, which is a saturated heterocycle that consisting of one nitrogen atom, two sulfur atoms at the 1-,3-, and 5- position, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azacyclic compounds |
|---|
| Sub Class | Dithiazinanes |
|---|
| Direct Parent | 1,3,5-dithiazinanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3,5-dithiazinane
- Thioacetal
- Dialkylthioether
- Hemithioaminal
- Thioether
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056u-9800000000-44297cffe9a5cf5e83be | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1290000000-56534006eac99beafb5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-8590000000-a4f511c8312b057eaaf4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-9200000000-d7cae298ba7a6e82d6e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3900000000-bbfc2b814ec1482610f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxr-8900000000-762257247e6c851cbee7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9000000000-9f35b06ff3a50ae04b25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-aa2b37dc911ec16015ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2590000000-f2a2932783d7cfdebeda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05vo-9200000000-6c791a40f91e7c6eb25e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0390000000-16060b2d048856f122ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-6940000000-8ad14ef8567142af8ac4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k92-9820000000-1610fc6c94e6adfd688c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040337 |
|---|
| FooDB ID | FDB020062 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014932 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 88622901 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|