| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:15:08 UTC |
|---|
| Update Date | 2016-11-09 01:20:56 UTC |
|---|
| Accession Number | CHEM033548 |
|---|
| Identification |
|---|
| Common Name | Dihydro-2,4-dimethyl-6-(2-methylpropyl)-4H-1,3,5-dithiazine |
|---|
| Class | Small Molecule |
|---|
| Description | Dihydro-2,4-dimethyl-6-(2-methylpropyl)-4H-1,3,5-dithiazine is found in mollusks. Dihydro-2,4-dimethyl-6-(2-methylpropyl)-4H-1,3,5-dithiazine is isolated from dried squid aroma. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
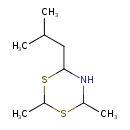
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C9H19NS2 |
|---|
| Average Molecular Mass | 205.384 g/mol |
|---|
| Monoisotopic Mass | 205.096 g/mol |
|---|
| CAS Registry Number | 101517-86-6 |
|---|
| IUPAC Name | 2,4-dimethyl-6-(2-methylpropyl)-1,3,5-dithiazinane |
|---|
| Traditional Name | 2,4-dimethyl-6-(2-methylpropyl)-1,3,5-dithiazinane |
|---|
| SMILES | CC(C)CC1NC(C)SC(C)S1 |
|---|
| InChI Identifier | InChI=1S/C9H19NS2/c1-6(2)5-9-10-7(3)11-8(4)12-9/h6-10H,5H2,1-4H3 |
|---|
| InChI Key | TWYULKMKVIUSSQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3,5-dithiazinanes. These are cyclic compounds that contain a dithiazinane ring, which is a saturated heterocycle that consisting of one nitrogen atom, two sulfur atoms at the 1-,3-, and 5- position, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azacyclic compounds |
|---|
| Sub Class | Dithiazinanes |
|---|
| Direct Parent | 1,3,5-dithiazinanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3,5-dithiazinane
- Thioacetal
- Dialkylthioether
- Hemithioaminal
- Thioether
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-8900000000-0c39ba735769335b3f46 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2190000000-a016beba12e1633447f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0900-6920000000-58c373a5d94de5937137 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pvi-9000000000-272dd836c78eabd5b411 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2900000000-e90d80cd3d0d5c2456ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0btc-9400000000-114449d81f8170d01506 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-9100000000-24767f482ee65723c102 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-9034b6d291e3d2b61946 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-5090000000-247e1cf0b7f763f57f6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-3131b3e4c5dc306b28b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-8c111527fc3978306881 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1490000000-3636944250bdbd97a5f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-9120000000-4b711c94af951e5a5fbb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040334 |
|---|
| FooDB ID | FDB020059 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 32699509 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 71587382 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|