| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:14:48 UTC |
|---|
| Update Date | 2016-11-09 01:20:56 UTC |
|---|
| Accession Number | CHEM033540 |
|---|
| Identification |
|---|
| Common Name | (7R*,8R*)-3-Methoxy-3',4,7,9,9'-pentahydroxy-8,4'-oxyneolignan 4-xyloside |
|---|
| Class | Small Molecule |
|---|
| Description | (7R*,8R*)-3-Methoxy-3',4,7,9,9'-pentahydroxy-8,4'-oxyneolignan 4-xyloside is a constituent of Pinus sylvestris (Scotch pine). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
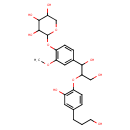
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C24H32O11 |
|---|
| Average Molecular Mass | 496.504 g/mol |
|---|
| Monoisotopic Mass | 496.194 g/mol |
|---|
| CAS Registry Number | 62223-56-7 |
|---|
| IUPAC Name | 2-(4-{1,3-dihydroxy-2-[2-hydroxy-4-(3-hydroxypropyl)phenoxy]propyl}-2-methoxyphenoxy)oxane-3,4,5-triol |
|---|
| Traditional Name | 2-(4-{1,3-dihydroxy-2-[2-hydroxy-4-(3-hydroxypropyl)phenoxy]propyl}-2-methoxyphenoxy)oxane-3,4,5-triol |
|---|
| SMILES | COC1=C(OC2OCC(O)C(O)C2O)C=CC(=C1)C(O)C(CO)OC1=C(O)C=C(CCCO)C=C1 |
|---|
| InChI Identifier | InChI=1S/C24H32O11/c1-32-19-10-14(5-7-18(19)35-24-23(31)22(30)16(28)12-33-24)21(29)20(11-26)34-17-6-4-13(3-2-8-25)9-15(17)27/h4-7,9-10,16,20-31H,2-3,8,11-12H2,1H3 |
|---|
| InChI Key | XBHWAKRDNVCHEC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lignan glycosides. These are aromatic polycyclic compounds containing a carbohydrate component glycosidically linked to a lignan moiety. They include 1-aryltetralin lactones. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Lignan glycosides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lignan glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Lignan glycoside
- Phenolic glycoside
- Glycosyl compound
- O-glycosyl compound
- Pentose monosaccharide
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Oxane
- Monosaccharide
- Monocyclic benzene moiety
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Acetal
- Oxacycle
- Ether
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Aromatic alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kk9-6760900000-c936f012f0c19dca55d7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-056r-5751439000-b5aa6bc0ed493c924db2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_19) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("(7R*,8R*)-3-Methoxy-3',4,7,9,9'-pentahydroxy-8,4'-oxyneolignan 4-xyloside,3TBDMS,#19" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00mk-0309800000-0cc8773f11585201dd04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0409100000-94f29382baf6ccac6d16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxs-1912000000-d0401da1e90ba3691c5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1314900000-6e62f79d4c91591583fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0924200000-79880c614f91cb2968af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014j-1900000000-7e28132ef2b2d098eb6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0109000000-1c9c69237e824c214667 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-2609300000-adaaf85e3ecbdc8a5014 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03ds-1922200000-454d59ccc7ed35e9178a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0113900000-27cbed75756e5cca4bbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0719500000-66ee5a628aae0ab736a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-1900000000-cb758ad64e9acfb6cd8a | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040325 |
|---|
| FooDB ID | FDB020048 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752793 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|