| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:12:15 UTC |
|---|
| Update Date | 2016-11-09 01:20:56 UTC |
|---|
| Accession Number | CHEM033483 |
|---|
| Identification |
|---|
| Common Name | 2,3,5,6,8,8a-Hexahydro-2,5,5,8a-tetramethyl-7H-1-benzopyran-7-one |
|---|
| Class | Small Molecule |
|---|
| Description | 2,3,5,6,8,8a-Hexahydro-2,5,5,8a-tetramethyl-7H-1-benzopyran-7-one is found in alcoholic beverages. 2,3,5,6,8,8a-Hexahydro-2,5,5,8a-tetramethyl-7H-1-benzopyran-7-one is a constituent of purple passion fruit, Riesling wine, grapes and grape leaf, quince, dried kukoshi berries (Lycium chinense) and sweet osmanthus (Osmanthus fragrans) flowers. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
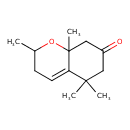
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-dihydro-3-Oxoedulan | HMDB |
|
|---|
| Chemical Formula | C13H20O2 |
|---|
| Average Molecular Mass | 208.297 g/mol |
|---|
| Monoisotopic Mass | 208.146 g/mol |
|---|
| CAS Registry Number | 20194-67-6 |
|---|
| IUPAC Name | 2,5,5,8a-tetramethyl-3,5,6,7,8,8a-hexahydro-2H-1-benzopyran-7-one |
|---|
| Traditional Name | 2,5,5,8a-tetramethyl-2,3,6,8-tetrahydro-1-benzopyran-7-one |
|---|
| SMILES | CC1CC=C2C(C)(C)CC(=O)CC2(C)O1 |
|---|
| InChI Identifier | InChI=1S/C13H20O2/c1-9-5-6-11-12(2,3)7-10(14)8-13(11,4)15-9/h6,9H,5,7-8H2,1-4H3 |
|---|
| InChI Key | PYUYJSFTZPYHDW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzopyrans. These are organic compounds containing a benzene ring fused to a pyran ring. Pyran a six-membered heterocyclic, non-aromatic ring, made up of five carbon atoms and one oxygen atom and containing two double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzopyran
- Pyran
- Cyclic ketone
- Ketone
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0wt9-1900000000-5fb27da915f579be0e4d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0490000000-1ba6f5b7812e077d7ac5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-3920000000-d14d417115aa1c9bde3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pbc-9600000000-619dd413880e79e55e69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-dfb742e2a3be2ceb4621 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0190000000-5c0936adb44d2f43b05c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9700000000-90867c9fccfa1e38da8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0590000000-9de79424715997bee799 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01z9-8900000000-5558f86d51bebaf3783d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-9800000000-81b30ef091d2e67c823b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-43a9bdbf7663e467c30d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-ac23213061ed0fcba5e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2920000000-19ee273a89e765e872fb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040233 |
|---|
| FooDB ID | FDB019948 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054418 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 470508 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 540316 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|