| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:12:12 UTC |
|---|
| Update Date | 2016-11-09 01:20:56 UTC |
|---|
| Accession Number | CHEM033482 |
|---|
| Identification |
|---|
| Common Name | Octahydro-2,5,5,8a-tetramethyl-7H-1-benzopyran-7-one |
|---|
| Class | Small Molecule |
|---|
| Description | Octahydro-2,5,5,8a-tetramethyl-7H-1-benzopyran-7-one is found in fruits. Octahydro-2,5,5,8a-tetramethyl-7H-1-benzopyran-7-one is a constituent of dried kukoshi berries (Lycium chinense) and tobacco oil. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
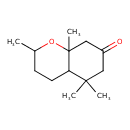
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3,7,7-Tetramethyl-2-oxabicyclo[4.4.0]decan-9-one | HMDB | | tetrahydro-3-Oxoedulan | HMDB |
|
|---|
| Chemical Formula | C13H22O2 |
|---|
| Average Molecular Mass | 210.313 g/mol |
|---|
| Monoisotopic Mass | 210.162 g/mol |
|---|
| CAS Registry Number | 5835-18-7 |
|---|
| IUPAC Name | 2,5,5,8a-tetramethyl-octahydro-2H-1-benzopyran-7-one |
|---|
| Traditional Name | 2,5,5,8a-tetramethyl-hexahydro-1-benzopyran-7-one |
|---|
| SMILES | CC1CCC2C(C)(C)CC(=O)CC2(C)O1 |
|---|
| InChI Identifier | InChI=1S/C13H22O2/c1-9-5-6-11-12(2,3)7-10(14)8-13(11,4)15-9/h9,11H,5-8H2,1-4H3 |
|---|
| InChI Key | VXIZSNZJQJVFMK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzopyrans. These are organic compounds containing a benzene ring fused to a pyran ring. Pyran a six-membered heterocyclic, non-aromatic ring, made up of five carbon atoms and one oxygen atom and containing two double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzopyran
- Oxane
- Cyclic ketone
- Ketone
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-1900000000-2a567d24808f31c66ca4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0590000000-bf289e62494f66e10105 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0cdi-5910000000-5638a01b04512fd57de1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-9600000000-848881e8ff348024c026 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-4fbe26010bea98022cce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-1960000000-23c92d1186fd3e5f6437 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-1003-7900000000-960e0b102a4d12369e80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0690000000-cd04378aadc2bb13d04d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-6910000000-63501f8556ff782fda96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053f-9500000000-56677d5553b68409a692 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-b989395baa8c25be3f80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-b989395baa8c25be3f80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-1930000000-192e67a9c44bbf911ffe | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040232 |
|---|
| FooDB ID | FDB019947 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057455 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 470098 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 539850 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|