| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:11:05 UTC |
|---|
| Update Date | 2016-11-09 01:20:55 UTC |
|---|
| Accession Number | CHEM033459 |
|---|
| Identification |
|---|
| Common Name | 2,6-Di-tert-butyl-1,4-benzenediol |
|---|
| Class | Small Molecule |
|---|
| Description | 2,6-Di-tert-butyl-1,4-benzenediol is found in tea. 2,6-Di-tert-butyl-1,4-benzenediol is isolated from ginseng roots. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
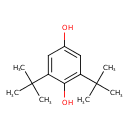
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 25-Ditertbutylhydroquinone | ChEMBL, HMDB | | 2,6-Bis(1,1-dimethylethyl)-1,4-benzenediol | HMDB | | 2,6-Bis(1,1-dimethylethyl)-1,4-benzenediol, 9ci | HMDB | | 2,6-Di-tert-butyl-hydroquinone | HMDB | | 2,6-Di-tert-butylbenzene-1,4-diol | HMDB | | 2,6-Di-tert-butylhydroquinone | HMDB |
|
|---|
| Chemical Formula | C14H22O2 |
|---|
| Average Molecular Mass | 222.323 g/mol |
|---|
| Monoisotopic Mass | 222.162 g/mol |
|---|
| CAS Registry Number | 2444-28-2 |
|---|
| IUPAC Name | 2,6-di-tert-butylbenzene-1,4-diol |
|---|
| Traditional Name | 2,6-di-tert-butylbenzene-1,4-diol |
|---|
| SMILES | CC(C)(C)C1=CC(O)=CC(=C1O)C(C)(C)C |
|---|
| InChI Identifier | InChI=1S/C14H22O2/c1-13(2,3)10-7-9(15)8-11(12(10)16)14(4,5)6/h7-8,15-16H,1-6H3 |
|---|
| InChI Key | JFGVTUJBHHZRAB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpropanes. These are organic compounds containing a phenylpropane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpropanes |
|---|
| Direct Parent | Phenylpropanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Hydroquinone
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-4980000000-36b3bfed874924700017 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0uk9-7189000000-f726627f6ca0a0827cd2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0190000000-0e1796c277b310ea27f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xr-1890000000-23ea378c3413481e08e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9410000000-01fa74bb66f8da9bc817 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-779b9de1b7f03218d290 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0290000000-13b061d618dbcfc8c915 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00m4-4920000000-9fb9520301a22051c806 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xr-2890000000-c6c5167adba8d520987f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9100000000-94de5d62011be4081020 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9300000000-b176fae324735b3ab715 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-277654a816c7fdb457aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-277654a816c7fdb457aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-2950000000-4457549f50228b4130ca | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0040178 |
|---|
| FooDB ID | FDB019890 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 68075 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 75550 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|