| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:10:20 UTC |
|---|
| Update Date | 2016-11-09 01:20:55 UTC |
|---|
| Accession Number | CHEM033445 |
|---|
| Identification |
|---|
| Common Name | N-Acetyllactosamine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
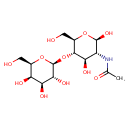
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| beta-D-Galactosyl-1,4-N-acetyl-D-glucosamine | ChEBI | | Galb1-4glcnacb | ChEBI | | Galbeta1-4glcnacbeta | ChEBI | | LacNAc | ChEBI | | N-Acetyl-beta-lactosamine | ChEBI | | beta-D-Galactosyl-1,4-N-acetyl-beta-D-glucosamine | Kegg | | b-D-Galactosyl-1,4-N-acetyl-D-glucosamine | Generator | | Β-D-galactosyl-1,4-N-acetyl-D-glucosamine | Generator | | N-Acetyl-b-lactosamine | Generator | | N-Acetyl-β-lactosamine | Generator | | b-D-Galactosyl-1,4-N-acetyl-b-D-glucosamine | Generator | | Β-D-galactosyl-1,4-N-acetyl-β-D-glucosamine | Generator | | beta-delta-Galactosyl-1,4-N-acetyl-beta-delta-glucosamine | HMDB | | beta-delta-Galactosyl-1,4-N-acetyl-delta-glucosamine | HMDB | | D-Galactopyranosyl | HMDB | | delta-Galactopyranosyl | HMDB | | N-Acetyl-lactosamine | HMDB | | ACETYL lactosamine | HMDB |
|
|---|
| Chemical Formula | C28H50N2O22 |
|---|
| Average Molecular Mass | 766.697 g/mol |
|---|
| Monoisotopic Mass | 766.286 g/mol |
|---|
| CAS Registry Number | 32181-59-2 |
|---|
| IUPAC Name | N-[(2R,3R,4R,5S,6R)-2,4-dihydroxy-6-(hydroxymethyl)-5-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-3-yl]acetamide |
|---|
| Traditional Name | N-acetyllactosamine |
|---|
| SMILES | CC(=O)NC(C=O)C(O)C(OC1OC(CO)C(O)C(O)C1O)C(O)CO.CC(=O)NC1C(O)OC(CO)C(OC2OC(CO)C(O)C(O)C2O)C1O |
|---|
| InChI Identifier | InChI=1S/2C14H25NO11/c1-4(18)15-7-9(20)12(6(3-17)24-13(7)23)26-14-11(22)10(21)8(19)5(2-16)25-14;1-5(19)15-6(2-16)9(21)13(7(20)3-17)26-14-12(24)11(23)10(22)8(4-18)25-14/h5-14,16-17,19-23H,2-3H2,1H3,(H,15,18);2,6-14,17-18,20-24H,3-4H2,1H3,(H,15,19) |
|---|
| InChI Key | DWPBOAJUXRQZDI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminosugar
- N-acyl-alpha-hexosamine
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Oxane
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Alcohol
- Primary alcohol
- Organonitrogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | - beta-D-galactopyranosyl-(1->4)-N-acetyl-D-glucosamine (CHEBI:16153 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uy3-5539000000-9001b4ffdf2686f61fdb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0a4i-3410139000-490a44b54a788af499ad | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0g4i-0294000000-eba3eabd1634e1f899d6 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-002r-0900000000-65d60bd6a084f5fb8a03 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0019-9800000000-5cfef19dd8c8707bef7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00yi-0179000000-d117b299438bee3a1a40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0592000000-db6f5abfab26c98d8af4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wbc-5960000000-915e1f050484eedb63d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-2492000000-1208b9128bdb1fd8688e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0h90-7984000000-2636a4af2a05389c39e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-7910000000-dce1bda7d8788ebd90af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-003r-1169000000-37af2930ba0a18fdfe2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bu3-9317000000-05676e3bb792b14a9ea9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9410000000-9f5dbeeb51b744740d72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f8a-0659000000-98f0c5a2f817d77728ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0951000000-f475151e03af91aeeac3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006w-9410000000-abd7ef88c6f0a3d44980 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001542 |
|---|
| FooDB ID | FDB019860 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 4231 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | N-Acetyllactosamine |
|---|
| Chemspider ID | 388404 |
|---|
| ChEBI ID | 16153 |
|---|
| PubChem Compound ID | 439271 |
|---|
| Kegg Compound ID | C00611 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005453 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Dekany, Gyula; Agoston, Karoly; Bajza, Istvan; Boejstrup, Marie; Kroeger, Lars. Process for the large-scale preparation of N-acetyllactosamine, lactosamine, lactosamine salts and lactosamine-containing oligosaccharides. PCT Int. Appl. (2007), 44pp. | | 2. Jung SK, Fujimoto D: A novel beta-galactoside-binding lectin in adult rat kidney. J Biochem. 1994 Sep;116(3):547-53. | | 3. Yu LC, Twu YC, Chang CY, Lin M: Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood. 2001 Dec 15;98(13):3840-5. | | 4. Dabelsteen E, Buschard K, Hakomori S, Young WW: Pattern of distribution of blood group antigens on human epidermal cells during maturation. J Invest Dermatol. 1984 Jan;82(1):13-7. | | 5. Ahmed H, Pohl J, Fink NE, Strobel F, Vasta GR: The primary structure and carbohydrate specificity of a beta-galactosyl-binding lectin from toad (Bufo arenarum Hensel) ovary reveal closer similarities to the mammalian galectin-1 than to the galectin from the clawed frog Xenopus laevis. J Biol Chem. 1996 Dec 20;271(51):33083-94. | | 6. Hosomi O, Takeya A: The relationship between the (beta 1-3) N-acetylglucosaminyltransferase and the presence of oligosaccharides containing lacto-N-triose II structure in bovine and human milk. Nihon Juigaku Zasshi. 1989 Feb;51(1):1-6. | | 7. Mollicone R, Candelier JJ, Mennesson B, Couillin P, Venot AP, Oriol R: Five specificity patterns of (1----3)-alpha-L-fucosyltransferase activity defined by use of synthetic oligosaccharide acceptors. Differential expression of the enzymes during human embryonic development and in adult tissues. Carbohydr Res. 1992 Apr 10;228(1):265-76. | | 8. Jinno K, Moriwaki S, Govindarajan S, Okada Y, Tsuji T: Blood group antigens in the intrahepatic biliary tree. II. Type 1 chain N-acetyllactosamine-related carbohydrate antigens in the proliferated bile ductules. J Hepatol. 1989 May;8(3):330-7. | | 9. Takeya A, Hosomi O, Kogure T: The presence of N-acetyllactosamine and lactose: beta (1-3)N-acetylglucosaminyltransferase activity in human urine. Jpn J Med Sci Biol. 1985 Feb;38(1):1-8. | | 10. Draberova L, Cerna H, Brodska H, Boubelik M, Watt SM, Stanners CP, Draber P: Soluble isoforms of CEACAM1 containing the A2 domain: increased serum levels in patients with obstructive jaundice and differences in 3-fucosyl-N-acetyl-lactosamine moiety. Immunology. 2000 Oct;101(2):279-87. | | 11. Kojima S, Eguchi H, Ookawara T, Fujiwara N, Yasuda J, Nakagawa K, Yamamura T, Suzuki K: Clostridium botulinum type A progenitor toxin binds to Intestine-407 cells via N-acetyllactosamine moiety. Biochem Biophys Res Commun. 2005 Jun 3;331(2):571-6. | | 12. Hosomi O, Takeya A, Kogure T: Human serum contains N-acetyllactosamine: beta 1-3 N-acetylglucosaminyltransferase activity. J Biochem. 1984 Jun;95(6):1655-9. | | 13. Dabelsteen E, Holbrook K, Clausen H, Hakomori S: Cell surface carbohydrate changes during embryonic and fetal skin development. J Invest Dermatol. 1986 Jul;87(1):81-5. | | 14. Argueso P, Spurr-Michaud S, Tisdale A, Gipson IK: Variation in the amount of T antigen and N-acetyllactosamine oligosaccharides in human cervical mucus secretions with the menstrual cycle. J Clin Endocrinol Metab. 2002 Dec;87(12):5641-8. | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=11181561 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=11429474 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=14631106 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=14672941 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=16966407 | | 20. https://www.ncbi.nlm.nih.gov/pubmed/?term=18280060 | | 21. https://www.ncbi.nlm.nih.gov/pubmed/?term=18849325 | | 22. https://www.ncbi.nlm.nih.gov/pubmed/?term=19443021 | | 23. https://www.ncbi.nlm.nih.gov/pubmed/?term=19486884 | | 24. https://www.ncbi.nlm.nih.gov/pubmed/?term=21496117 | | 25. https://www.ncbi.nlm.nih.gov/pubmed/?term=2457603 | | 26. https://www.ncbi.nlm.nih.gov/pubmed/?term=25568069 | | 27. https://www.ncbi.nlm.nih.gov/pubmed/?term=26802542 | | 28. https://www.ncbi.nlm.nih.gov/pubmed/?term=31537530 | | 29. https://www.ncbi.nlm.nih.gov/pubmed/?term=7706263 | | 30. https://www.ncbi.nlm.nih.gov/pubmed/?term=8737251 |
|
|---|