| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:04:02 UTC |
|---|
| Update Date | 2016-11-09 01:20:53 UTC |
|---|
| Accession Number | CHEM033297 |
|---|
| Identification |
|---|
| Common Name | 2-Acetyl-3,5-dimethylpyrazine |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Acetyl-3,5-dimethylpyrazine is found in coffee and coffee products. 2-Acetyl-3,5-dimethylpyrazine is a constituent of wood smoke and coffee aroma. 2-Acetyl-3,5-dimethylpyrazine is a component of *FEMA 3327*. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
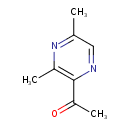
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(3,5-Dimethyl-2-pyrazinyl)-1-ethanone | HMDB | | 1-(3,5-Dimethyl-2-pyrazinyl)-ethanone | HMDB | | 1-(3,5-Dimethyl-2-pyrazinyl)ethanone | HMDB | | 1-(3,5-Dimethylpyrazinyl)-ethanone | HMDB | | 1-(3,5-Dimethylpyrazinyl)ethan-1-one | HMDB | | 1-(3,5-Dimethylpyrazinyl)ethanone, 9ci | HMDB | | 2-Acetyl-3,5(6)-dimethylpyrazine | HMDB | | Pyrazine, 2-acetyl-3,5-dimethyl | HMDB |
|
|---|
| Chemical Formula | C8H10N2O |
|---|
| Average Molecular Mass | 150.178 g/mol |
|---|
| Monoisotopic Mass | 150.079 g/mol |
|---|
| CAS Registry Number | 54300-08-2 |
|---|
| IUPAC Name | 1-(3,5-dimethylpyrazin-2-yl)ethan-1-one |
|---|
| Traditional Name | 1-(3,5-dimethylpyrazin-2-yl)ethanone |
|---|
| SMILES | CC(=O)C1=C(C)N=C(C)C=N1 |
|---|
| InChI Identifier | InChI=1S/C8H10N2O/c1-5-4-9-8(7(3)11)6(2)10-5/h4H,1-3H3 |
|---|
| InChI Key | UCGOSAWBWFUKDT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryl alkyl ketones. These are ketones have the generic structure RC(=O)R', where R = aryl group and R'=alkyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Aryl alkyl ketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aryl alkyl ketone
- Pyrazine
- Heteroaromatic compound
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-6900000000-cac66b25398f62410a08 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-770f712e5e826c2cb5f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0900000000-bd35f79cd5aec49eb77a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015c-9400000000-c0690f8a09ab4c0487f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-7fb0b85f4419fb6552e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-0900000000-fa75afabd22785703de3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5c-8900000000-1f72bc76f0a0a320bda5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-150ccace3c6c27ddc9f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-4900000000-05bc86e280892864bb33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-9100000000-ee126ebfe463424b1783 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-d465dda09117a78fa7bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9700000000-f6dbd290136e93256f4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07bf-9300000000-bc245a9708b9adf96374 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039999 |
|---|
| FooDB ID | FDB019681 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 94540 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 104721 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|