| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:02:47 UTC |
|---|
| Update Date | 2016-11-09 01:20:53 UTC |
|---|
| Accession Number | CHEM033269 |
|---|
| Identification |
|---|
| Common Name | (1R,3R,4R,5S,6S,8x)-1-Acetoxy-8-angeloyloxy-3,4-epoxy-5-hydroxy-7(14),10-bisaboladien-2-one |
|---|
| Class | Small Molecule |
|---|
| Description | (1R,3R,4R,5S,6S,8x)-1-Acetoxy-8-angeloyloxy-3,4-epoxy-5-hydroxy-7(14),10-bisaboladien-2-one is found in tea. (1R,3R,4R,5S,6S,8x)-1-Acetoxy-8-angeloyloxy-3,4-epoxy-5-hydroxy-7(14),10-bisaboladien-2-one is a constituent of flower buds of Tussilago farfara (coltsfoot). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
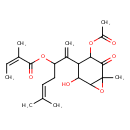
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ulithiacyclamide | HMDB | | 2-[4-(Acetyloxy)-2-hydroxy-6-methyl-5-oxo-7-oxabicyclo[4.1.0]heptan-3-yl]-6-methylhepta-1,5-dien-3-yl (2Z)-2-methylbut-2-enoic acid | Generator |
|
|---|
| Chemical Formula | C22H30O7 |
|---|
| Average Molecular Mass | 406.469 g/mol |
|---|
| Monoisotopic Mass | 406.199 g/mol |
|---|
| CAS Registry Number | 348113-03-1 |
|---|
| IUPAC Name | 2-[4-(acetyloxy)-2-hydroxy-6-methyl-5-oxo-7-oxabicyclo[4.1.0]heptan-3-yl]-6-methylhepta-1,5-dien-3-yl (2Z)-2-methylbut-2-enoate |
|---|
| Traditional Name | 2-[4-(acetyloxy)-2-hydroxy-6-methyl-5-oxo-7-oxabicyclo[4.1.0]heptan-3-yl]-6-methylhepta-1,5-dien-3-yl (2Z)-2-methylbut-2-enoate |
|---|
| SMILES | C\C=C(\C)C(=O)OC(CC=C(C)C)C(=C)C1C(O)C2OC2(C)C(=O)C1OC(C)=O |
|---|
| InChI Identifier | InChI=1S/C22H30O7/c1-8-12(4)21(26)28-15(10-9-11(2)3)13(5)16-17(24)20-22(7,29-20)19(25)18(16)27-14(6)23/h8-9,15-18,20,24H,5,10H2,1-4,6-7H3/b12-8- |
|---|
| InChI Key | NTVBUJOKRXFMAK-WQLSENKSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Bisabolane sesquiterpenoid
- Fatty acid ester
- Oxepane
- Alpha-acyloxy ketone
- Fatty acyl
- Dicarboxylic acid or derivatives
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid ester
- Ketone
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Oxirane
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9123000000-3a83847a45edb0df5166 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-9202100000-b427b90aebebb79d9b80 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-3019300000-651f6e4aa4a2ec78afbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a59-9134000000-f52f5096d8ac277c7343 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9010000000-1e4531c74a03f0e6e1bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-5019700000-29ae9d439f6961049f1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-8129100000-d574eca8aa43271668f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9122000000-fc20b0fcf809f9e4c58d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-9011300000-a5921d1efa8c5eea0664 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-9023100000-87b9475cd2a30ca5d327 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-9100000000-3db0d1afcd0f93bb665a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052b-0094100000-35a7052abd7884eb1f1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0195300000-67162f72585713b9a472 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9431000000-c0f1bd1873242ac798c0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039971 |
|---|
| FooDB ID | FDB019639 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014909 |
|---|
| ChEBI ID | 171847 |
|---|
| PubChem Compound ID | 131752774 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|