| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:02:39 UTC |
|---|
| Update Date | 2016-11-09 01:20:53 UTC |
|---|
| Accession Number | CHEM033266 |
|---|
| Identification |
|---|
| Common Name | Brevetoxin B4b |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from Perna canaliculus (New Zealand green mussel). Brevetoxin B4b is found in mollusks. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
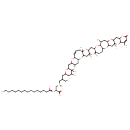
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| BTXB4b | HMDB | | 3-(3-Hydroxy-2-{[(21Z)-12-hydroxy-1,3,11,24,31,41,44-heptamethyl-39-oxo-2,6,10,15,19,25,29,34,38,43,47-undecaoxaundecacyclo[26.22.0.0³,²⁶.0⁵,²⁴.0⁷,²⁰.0⁹,¹⁸.0¹¹,¹⁶.0³⁰,⁴⁸.0³³,⁴⁶.0³⁵,⁴⁴.0³⁷,⁴²]pentaconta-21,40-dien-14-yl]methyl}propanesulfinyl)-2-[(1-hydroxyhexadecylidene)amino]propanoate | Generator | | 3-(3-Hydroxy-2-{[(21Z)-12-hydroxy-1,3,11,24,31,41,44-heptamethyl-39-oxo-2,6,10,15,19,25,29,34,38,43,47-undecaoxaundecacyclo[26.22.0.0³,²⁶.0⁵,²⁴.0⁷,²⁰.0⁹,¹⁸.0¹¹,¹⁶.0³⁰,⁴⁸.0³³,⁴⁶.0³⁵,⁴⁴.0³⁷,⁴²]pentaconta-21,40-dien-14-yl]methyl}propanesulphinyl)-2-[(1-hydroxyhexadecylidene)amino]propanoate | Generator | | 3-(3-Hydroxy-2-{[(21Z)-12-hydroxy-1,3,11,24,31,41,44-heptamethyl-39-oxo-2,6,10,15,19,25,29,34,38,43,47-undecaoxaundecacyclo[26.22.0.0³,²⁶.0⁵,²⁴.0⁷,²⁰.0⁹,¹⁸.0¹¹,¹⁶.0³⁰,⁴⁸.0³³,⁴⁶.0³⁵,⁴⁴.0³⁷,⁴²]pentaconta-21,40-dien-14-yl]methyl}propanesulphinyl)-2-[(1-hydroxyhexadecylidene)amino]propanoic acid | Generator |
|
|---|
| Chemical Formula | C69H109NO18S |
|---|
| Average Molecular Mass | 1272.665 g/mol |
|---|
| Monoisotopic Mass | 1271.737 g/mol |
|---|
| CAS Registry Number | 260270-44-8 |
|---|
| IUPAC Name | 3-(3-hydroxy-2-{[(21Z)-12-hydroxy-1,3,11,24,31,41,44-heptamethyl-39-oxo-2,6,10,15,19,25,29,34,38,43,47-undecaoxaundecacyclo[26.22.0.0³,²⁶.0⁵,²⁴.0⁷,²⁰.0⁹,¹⁸.0¹¹,¹⁶.0³⁰,⁴⁸.0³³,⁴⁶.0³⁵,⁴⁴.0³⁷,⁴²]pentaconta-21,40-dien-14-yl]methyl}propanesulfinyl)-2-[(Z)-(1-hydroxyhexadecylidene)amino]propanoic acid |
|---|
| Traditional Name | 3-(3-hydroxy-2-{[(21Z)-12-hydroxy-1,3,11,24,31,41,44-heptamethyl-39-oxo-2,6,10,15,19,25,29,34,38,43,47-undecaoxaundecacyclo[26.22.0.0³,²⁶.0⁵,²⁴.0⁷,²⁰.0⁹,¹⁸.0¹¹,¹⁶.0³⁰,⁴⁸.0³³,⁴⁶.0³⁵,⁴⁴.0³⁷,⁴²]pentaconta-21,40-dien-14-yl]methyl}propanesulfinyl)-2-[(Z)-(1-hydroxyhexadecylidene)amino]propanoic acid |
|---|
| SMILES | CCCCCCCCCCCCCCC\C(O)=N\C(CS(=O)CC(CO)CC1CC(O)C2(C)OC3CC4OC5CC6(C)OC7(C)CCC8OC9CC%10(C)OC%11C(CC%10OC9CC(C)C8OC7CC6OC5(C)C\C=C/C4OC3CC2O1)OC(=O)C=C%11C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C69H109NO18S/c1-9-10-11-12-13-14-15-16-17-18-19-20-21-24-60(73)70-45(64(75)76)40-89(77)39-43(38-71)30-44-31-54(72)69(8)58(78-44)33-50-51(85-69)32-49-46(79-50)23-22-26-65(4)59(82-49)37-68(7)57(86-65)35-56-66(5,88-68)27-25-47-62(84-56)41(2)28-48-53(80-47)36-67(6)55(81-48)34-52-63(87-67)42(3)29-61(74)83-52/h22-23,29,41,43-59,62-63,71-72H,9-21,24-28,30-40H2,1-8H3,(H,70,73)(H,75,76)/b23-22- |
|---|
| InChI Key | SZLQDAWKHKBNOD-FCQUAONHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as brevetoxins and derivatives. These are a group of cyclic polyether compounds produced naturally by a species of dinoflagellate known as Karenia brevis. They contain a Pentaoxapentacycloheptacos- 23-en-7-one derivative (type a brevetoxin) or a pentaoxapentacyclotetracosa- 8,23-dien-7-one derivative (type b brevetoxin). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Brevetoxins and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Brevetoxins and derivatives |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2090000001-2dc1dfc05b9d93079dd6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gw1-5292100033-5443e6ab977af8518a50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03el-3091020043-59c1a2a5b4aa214804fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1094200100-91ad603e6a39d362d5fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ab9-1497075002-9993e68e215ec57a1dba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0avi-0395241210-543db7a10eaa704d14d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-1010000090-609c52e6719ee155751a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00e9-2190000040-3d6d95c00c7561a13659 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00as-1792001061-4ec191bb1e4f504d1fc2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0091000021-fcc61aa7f570a2dae90d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-2190000041-85ff02df5fa026cd79a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06y6-3950000020-8682630cbf04e20756bc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039968 |
|---|
| FooDB ID | FDB019635 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752772 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|