| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:02:14 UTC |
|---|
| Update Date | 2016-11-09 01:20:52 UTC |
|---|
| Accession Number | CHEM033255 |
|---|
| Identification |
|---|
| Common Name | 3-O-Caffeoyl-1-O-methylquinic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 3-O-Caffeoyl-1-O-methylquinic acid is found in green vegetables. It is a constituent of Phyllostachys edulis (moso bamboo). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
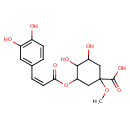
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-O-Caffeoyl-1-O-methylquinate | Generator | | 3-{[(2Z)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-4,5-dihydroxy-1-methoxycyclohexane-1-carboxylate | HMDB |
|
|---|
| Chemical Formula | C17H20O9 |
|---|
| Average Molecular Mass | 368.335 g/mol |
|---|
| Monoisotopic Mass | 368.111 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-{[(2Z)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-4,5-dihydroxy-1-methoxycyclohexane-1-carboxylic acid |
|---|
| Traditional Name | 3-{[(2Z)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-4,5-dihydroxy-1-methoxycyclohexane-1-carboxylic acid |
|---|
| SMILES | COC1(CC(O)C(O)C(C1)OC(=O)\C=C/C1=CC(O)=C(O)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C17H20O9/c1-25-17(16(23)24)7-12(20)15(22)13(8-17)26-14(21)5-3-9-2-4-10(18)11(19)6-9/h2-6,12-13,15,18-20,22H,7-8H2,1H3,(H,23,24)/b5-3- |
|---|
| InChI Key | NRRBIAVXVIGMKC-HYXAFXHYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinic acids and derivatives. Quinic acids and derivatives are compounds containing a quinic acid moiety (or a derivative thereof), which is a cyclitol made up of a cyclohexane ring that bears four hydroxyl groups at positions 1,3.4, and 5, as well as a carboxylic acid at position 1. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Quinic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinic acid
- Cinnamic acid or derivatives
- Coumaric acid or derivatives
- Hydroxycinnamic acid or derivatives
- Cinnamic acid ester
- Catechol
- Styrene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Cyclohexanol
- Fatty acid ester
- Phenol
- Benzenoid
- Fatty acyl
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Secondary alcohol
- 1,2-diol
- Carboxylic acid ester
- Dialkyl ether
- Carboxylic acid
- Ether
- Carboxylic acid derivative
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f7c-5914000000-94d83e8906e928f27c32 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-052f-4235029000-ce778b9dea2f92469ca8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0i00-0829000000-b9ecf6883ffa4b0ec7b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01w0-0912000000-433196924e25e78e3a0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-1900000000-3daa0e325b37b9384b0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0419000000-21b7427b8368c48277fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0770-0924000000-5404e6d60e9ea9616d26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08g0-0900000000-dab3f0d2168f5e1181dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0309000000-24a7e4146e6fe76b0e27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p6-0987000000-13efddacfbf3d2604674 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-090a-3900000000-0a03a2af07ec1de05ca1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0139000000-4758e372c670fce24b88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-0977000000-33a6908ad767450d0142 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-1932000000-9ec863aae7f11497b62e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039959 |
|---|
| FooDB ID | FDB019622 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014902 |
|---|
| ChEBI ID | 175715 |
|---|
| PubChem Compound ID | 131752768 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|