| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:01:50 UTC |
|---|
| Update Date | 2016-11-09 01:20:52 UTC |
|---|
| Accession Number | CHEM033244 |
|---|
| Identification |
|---|
| Common Name | 3,4-Dihydroxy-2-hydroxymethyl-1-pyrrolidinepropanamide |
|---|
| Class | Small Molecule |
|---|
| Description | 3,4-Dihydroxy-2-hydroxymethyl-1-pyrrolidinepropanamide is found in fruits. 3,4-Dihydroxy-2-hydroxymethyl-1-pyrrolidinepropanamide is an alkaloid from Morus alba (white mulberry). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
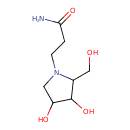
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-[3,4-Dihydroxy-2-(hydroxymethyl)pyrrolidin-1-yl]propanimidate | HMDB |
|
|---|
| Chemical Formula | C8H16N2O4 |
|---|
| Average Molecular Mass | 204.224 g/mol |
|---|
| Monoisotopic Mass | 204.111 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-[3,4-dihydroxy-2-(hydroxymethyl)pyrrolidin-1-yl]propanamide |
|---|
| Traditional Name | 3-[3,4-dihydroxy-2-(hydroxymethyl)pyrrolidin-1-yl]propanamide |
|---|
| SMILES | NC(=O)CCN1CC(O)C(O)C1CO |
|---|
| InChI Identifier | InChI=1S/C8H16N2O4/c9-7(13)1-2-10-3-6(12)8(14)5(10)4-11/h5-6,8,11-12,14H,1-4H2,(H2,9,13) |
|---|
| InChI Key | ARJIGPTXXUGWDZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-alkylpyrrolidines. N-alkylpyrrolidines are compounds containing a pyrrolidine moiety that is substituted at the N1-position with an alkyl group. Pyrrolidine is a five-membered saturated aliphatic heterocycle with one nitrogen atom and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolidines |
|---|
| Sub Class | N-alkylpyrrolidines |
|---|
| Direct Parent | N-alkylpyrrolidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-alkylpyrrolidine
- 1,2-aminoalcohol
- Secondary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Carboximidic acid
- Carboximidic acid derivative
- Azacycle
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic nitrogen compound
- Amine
- Organopnictogen compound
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006x-6900000000-174176d1b422ac2870da | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-4149100000-1fe3d9b6d07fff1bd23e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0930000000-75e617af7f5912bc72df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00y0-2900000000-17d1aae137479073ff93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01b9-8900000000-981690fa15a183f3d848 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uk9-1950000000-66f1699325dac28951cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0076-4900000000-1375760e264763e17568 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-d1f3c3a909ada5863a98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0790000000-b874d2edffc673e1f9f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ar9-1910000000-d5589451a5c18cf4de1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ac3-9300000000-52763d92421a47fae72c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0920000000-663e80e126b30765deee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w2c-7920000000-7cab85a374b39cf08dc8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-54fb86e1fa083f85feff | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039948 |
|---|
| FooDB ID | FDB019610 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014900 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 85303757 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|