| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:01:04 UTC |
|---|
| Update Date | 2016-11-09 01:20:52 UTC |
|---|
| Accession Number | CHEM033225 |
|---|
| Identification |
|---|
| Common Name | (-)-Lyoniresinol 9'-sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Lyoniresinol 9'-sulfate is found in green vegetables. Lyoniresinol 9'-sulfate is a constituent of the roots of Polygonum cuspidatum (Japanese knotweed). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
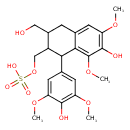
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Lyoniresinol 9'-sulfuric acid | Generator | | Lyoniresinol 9'-sulphate | Generator | | Lyoniresinol 9'-sulphuric acid | Generator | | {[7-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-6,8-dimethoxy-1,2,3,4-tetrahydronaphthalen-2-yl]methoxy}sulfonate | HMDB | | {[7-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-6,8-dimethoxy-1,2,3,4-tetrahydronaphthalen-2-yl]methoxy}sulphonate | HMDB | | {[7-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-6,8-dimethoxy-1,2,3,4-tetrahydronaphthalen-2-yl]methoxy}sulphonic acid | HMDB | | (-)-Lyoniresinol 9'-sulfuric acid | HMDB | | (-)-Lyoniresinol 9'-sulphate | HMDB | | (-)-Lyoniresinol 9'-sulphuric acid | HMDB |
|
|---|
| Chemical Formula | C22H28O11S |
|---|
| Average Molecular Mass | 500.516 g/mol |
|---|
| Monoisotopic Mass | 500.135 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[7-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-6,8-dimethoxy-1,2,3,4-tetrahydronaphthalen-2-yl]methoxy}sulfonic acid |
|---|
| Traditional Name | [7-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-6,8-dimethoxy-1,2,3,4-tetrahydronaphthalen-2-yl]methoxysulfonic acid |
|---|
| SMILES | COC1=CC(=CC(OC)=C1O)C1C(COS(O)(=O)=O)C(CO)CC2=CC(OC)=C(O)C(OC)=C12 |
|---|
| InChI Identifier | InChI=1S/C22H28O11S/c1-29-15-7-12(8-16(30-2)20(15)24)18-14(10-33-34(26,27)28)13(9-23)5-11-6-17(31-3)21(25)22(32-4)19(11)18/h6-8,13-14,18,23-25H,5,9-10H2,1-4H3,(H,26,27,28) |
|---|
| InChI Key | RRBNWWDPKOJFOA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryltetralin lignans. These are lignans with a structure based on the 1-phenyltetralin skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Aryltetralin lignans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aryltetralin lignans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-aryltetralin lignan
- Methoxyphenol
- Tetralin
- Dimethoxybenzene
- M-dimethoxybenzene
- Methoxybenzene
- Anisole
- Phenol ether
- Phenoxy compound
- Phenol
- Alkyl aryl ether
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Benzenoid
- Monocyclic benzene moiety
- Alkyl sulfate
- Organic sulfuric acid or derivatives
- Ether
- Primary alcohol
- Organic oxygen compound
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014r-0103900000-b84f4ddc8eca5bd343b9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-020r-1000139000-46caed72ec158f8eaecb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0002950000-bbbf04b749deee8b7627 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uei-1116900000-4c761f9bffe8a6c0248a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0lk9-0309500000-2dd1e2bd58c8a67ff310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1000900000-07ad62dbd0683ce527a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f7k-2000900000-27e171c8c228ba60b7dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uea-5003900000-a34d7d993b7ea0fdccd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0009410000-35cc210dae7e9ffe65f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uki-0049400000-4118a9b7b2ce955b7bbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ds-0049100000-300de83a34f8bc35950a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-a925d39acd554b951d01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fr2-1000900000-113cf7df02fd6c58dd0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-2008900000-44f6b9bb68f6bb1c1004 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039926 |
|---|
| FooDB ID | FDB019588 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014899 |
|---|
| ChEBI ID | 168801 |
|---|
| PubChem Compound ID | 74083657 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|