| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:00:07 UTC |
|---|
| Update Date | 2016-11-09 01:20:52 UTC |
|---|
| Accession Number | CHEM033202 |
|---|
| Identification |
|---|
| Common Name | Mangostenone B |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of the green fruit hulls of Garcinia mangostana (mangosteen). Mangostenone B is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
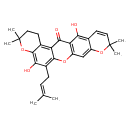
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Mangostenone b | MeSH |
|
|---|
| Chemical Formula | C28H30O6 |
|---|
| Average Molecular Mass | 462.534 g/mol |
|---|
| Monoisotopic Mass | 462.204 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 10,22-dihydroxy-7,7,18,18-tetramethyl-11-(3-methylbut-2-en-1-yl)-8,13,17-trioxapentacyclo[12.8.0.0³,¹².0⁴,⁹.0¹⁶,²¹]docosa-1(14),3,9,11,15,19,21-heptaen-2-one |
|---|
| Traditional Name | 10,22-dihydroxy-7,7,18,18-tetramethyl-11-(3-methylbut-2-en-1-yl)-8,13,17-trioxapentacyclo[12.8.0.0³,¹².0⁴,⁹.0¹⁶,²¹]docosa-1(14),3,9,11,15,19,21-heptaen-2-one |
|---|
| SMILES | CC(C)=CCC1=C2OC3=C(C(O)=C4C=CC(C)(C)OC4=C3)C(=O)C2=C2CCC(C)(C)OC2=C1O |
|---|
| InChI Identifier | InChI=1S/C28H30O6/c1-14(2)7-8-17-23(30)26-16(10-12-28(5,6)34-26)20-24(31)21-19(32-25(17)20)13-18-15(22(21)29)9-11-27(3,4)33-18/h7,9,11,13,29-30H,8,10,12H2,1-6H3 |
|---|
| InChI Key | CTTYZRNOKDZJRY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4-prenylated xanthones. These are organic compounds containing a C5-isoprenoid group linked to a xanthone moiety at the 4-position. Xanthone is a tricyclic compound made up of two benzene rings linearly fused to each other through a pyran ring that carries a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 4-prenylated xanthones |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-2011900000-a1c394403d14e6b3f1a0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0006-1000090000-bfa1a0e60c51dcafae5a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-444578f7440af57e30c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2006900000-0fd0a8112c644e9ac501 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-6019300000-8e953a582a69aeb02dae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-46e16c73689940b65f51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-0002900000-ebe62ea5c4b3b7eb0729 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-1039200000-8cfbbbca2e75086abeae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-58e26f8f0b80f4864bc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000900000-f71df7ff5c3a85f3de21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gc3-0121900000-16cd7bde1fe27b501614 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fr-0000900000-9e73a5432243cf1ccead | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0001900000-49c52eac3b564a6f6a69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0abc-2109700000-24ca0be7b040c1ec6296 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039903 |
|---|
| FooDB ID | FDB019564 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10282248 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 21672078 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|