| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:59:52 UTC |
|---|
| Update Date | 2016-11-09 01:20:52 UTC |
|---|
| Accession Number | CHEM033195 |
|---|
| Identification |
|---|
| Common Name | xi-8-Acetonyldihydrosanguinarine |
|---|
| Class | Small Molecule |
|---|
| Description | xi-8-Acetonyldihydrosanguinarine is an alkaloid from Papaver somniferum (opium poppy). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
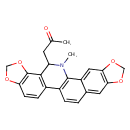
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Acetonyl-5,6-dihydrosanguinarine | Kegg | | (+/-)-8-acetonyldihydrosanguinarine | Kegg |
|
|---|
| Chemical Formula | C23H19NO5 |
|---|
| Average Molecular Mass | 389.401 g/mol |
|---|
| Monoisotopic Mass | 389.126 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 1-{24-methyl-5,7,18,20-tetraoxa-24-azahexacyclo[11.11.0.0²,¹⁰.0⁴,⁸.0¹⁴,²².0¹⁷,²¹]tetracosa-1(13),2,4(8),9,11,14(22),15,17(21)-octaen-23-yl}propan-2-one |
|---|
| Traditional Name | 1-{24-methyl-5,7,18,20-tetraoxa-24-azahexacyclo[11.11.0.0²,¹⁰.0⁴,⁸.0¹⁴,²².0¹⁷,²¹]tetracosa-1(13),2,4(8),9,11,14(22),15,17(21)-octaen-23-yl}propan-2-one |
|---|
| SMILES | CN1C(CC(C)=O)C2=C(C=CC3=C2OCO3)C2=C1C1=CC3=C(OCO3)C=C1C=C2 |
|---|
| InChI Identifier | InChI=1S/C23H19NO5/c1-12(25)7-17-21-14(5-6-18-23(21)29-11-26-18)15-4-3-13-8-19-20(28-10-27-19)9-16(13)22(15)24(17)2/h3-6,8-9,17H,7,10-11H2,1-2H3 |
|---|
| InChI Key | ONEHMWWDDDSJBB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydrobenzophenanthridine alkaloids. These are alkaloids containing a dihydrobenzophenanthridine skeleton, which is a tetracyclic compound containing a benzene fused to a dihydrophenanthridine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Benzophenanthridine alkaloids |
|---|
| Sub Class | Dihydrobenzophenanthridine alkaloids |
|---|
| Direct Parent | Dihydrobenzophenanthridine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydrobenzophenanthridine alkaloid skeleton
- Benzoquinoline
- Phenanthridine
- Naphthalene
- Quinoline
- Benzodioxole
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Aralkylamine
- Benzenoid
- Beta-aminoketone
- Tertiary amine
- Ketone
- Organoheterocyclic compound
- Acetal
- Oxacycle
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Organopnictogen compound
- Carbonyl group
- Organooxygen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-2009000000-364461530c94110463f8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0009000000-a9210693e08bd461c7b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-1009000000-95dab7d75068b2d63dce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fvl-4019000000-28c1e23170e0d65c1640 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-4f199831f3ef8da2d6bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-188316860990b073b6e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00tf-1009000000-a9270dd1276ced96622e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-3dc74d1aa25b63a7b857 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-2f1800b39acde55ecebb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ap3-0009000000-47a254e9b6837d9e9781 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-d9b57af363c8fd7af69a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0009000000-0d2c364b894aafb08ec0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbc-0009000000-b73a932dc1997d01ff0f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039896 |
|---|
| FooDB ID | FDB019556 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024626 C00027777 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 157916 |
|---|
| ChEBI ID | 31022 |
|---|
| PubChem Compound ID | 181538 |
|---|
| Kegg Compound ID | C12201 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|