| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:59:21 UTC |
|---|
| Update Date | 2016-11-09 01:20:52 UTC |
|---|
| Accession Number | CHEM033184 |
|---|
| Identification |
|---|
| Common Name | 3-Hydroxyneogrifolin |
|---|
| Class | Small Molecule |
|---|
| Description | 3-Hydroxyneogrifolin is found in mushrooms. 3-Hydroxyneogrifolin is a constituent of Albatrellus ovinus. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
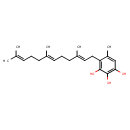
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Methyl-4-(3,7,11-trimethyl-2,6,10-dodecatrienyl)-1,2,3-benzenetriol | HMDB |
|
|---|
| Chemical Formula | C22H32O3 |
|---|
| Average Molecular Mass | 344.488 g/mol |
|---|
| Monoisotopic Mass | 344.235 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-methyl-4-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl]benzene-1,2,3-triol |
|---|
| Traditional Name | 5-methyl-4-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl]benzene-1,2,3-triol |
|---|
| SMILES | CC(C)=CCC\C(C)=C\CC\C(C)=C\CC1=C(O)C(O)=C(O)C=C1C |
|---|
| InChI Identifier | InChI=1S/C22H32O3/c1-15(2)8-6-9-16(3)10-7-11-17(4)12-13-19-18(5)14-20(23)22(25)21(19)24/h8,10,12,14,23-25H,6-7,9,11,13H2,1-5H3/b16-10+,17-12+ |
|---|
| InChI Key | BACDZNLMIXNCOG-JTCWOHKRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Farsesane sesquiterpenoid
- Sesquiterpenoid
- Benzenetriol
- Pyrogallol derivative
- M-cresol
- P-cresol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Toluene
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Polyol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0570-4973000000-913cb9dad6d632292f74 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0002-3300690000-60195216ab819e7a9fe6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0419000000-e586c3725ba3c189f67a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-106r-2921000000-77a8a24211da7dd825cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9750000000-c369980e7fe3a14ded22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-bee88434edf387865b0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0119000000-974e994d037ce72c884d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-2962000000-e63a89a156f8940ba1e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1936000000-5fae4eba63743590c7ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-4921000000-3b729689b5ef34456291 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-6910000000-44b4826007811a584934 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-ac6607003a942882fb06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0519000000-845aace38b3aa9405f25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-2930000000-b5977fb8ccf91507c76c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039885 |
|---|
| FooDB ID | FDB019544 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00036550 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4476903 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5318298 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|