| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:58:26 UTC |
|---|
| Update Date | 2016-11-09 01:20:51 UTC |
|---|
| Accession Number | CHEM033161 |
|---|
| Identification |
|---|
| Common Name | Cadabicine methyl ether |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
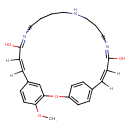
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C26H31N3O4 |
|---|
| Average Molecular Mass | 449.551 g/mol |
|---|
| Monoisotopic Mass | 449.231 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (8E,22E)-4-methoxy-2-oxa-11,16,20-triazatricyclo[22.2.2.1³,⁷]nonacosa-1(26),3,5,7(29),8,10,20,22,24,27-decaene-10,21-diol |
|---|
| Traditional Name | (8E,22E)-4-methoxy-2-oxa-11,16,20-triazatricyclo[22.2.2.1³,⁷]nonacosa-1(26),3,5,7(29),8,10,20,22,24,27-decaene-10,21-diol |
|---|
| SMILES | [H]\C1=C([H])\C(O)=NCCCNCCCCN=C(O)\C([H])=C([H])/C2=CC(OC3=CC=C1C=C3)=C(OC)C=C2 |
|---|
| InChI Identifier | InChI=1S/C26H31N3O4/c1-32-23-12-7-21-9-14-26(31)28-17-3-2-15-27-16-4-18-29-25(30)13-8-20-5-10-22(11-6-20)33-24(23)19-21/h5-14,19,27H,2-4,15-18H2,1H3,(H,28,31)(H,29,30)/b13-8-,14-9- |
|---|
| InChI Key | IXYCICGPUOJVOW-QKPPEBSVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as macrolactams. These are cyclic amides of amino carboxylic acids, having a 1-azacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. They are nitrogen analogues (the a nitrogen atom replacing the o atom of the cyclic carboxylic acid group ) of the naturally occurring macrolides. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolactams |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Macrolactams |
|---|
| Alternative Parents | |
|---|
| Substituents | - Macrolactam
- Diaryl ether
- Anisole
- Alkyl aryl ether
- Benzenoid
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Amino acid or derivatives
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Ether
- Secondary aliphatic amine
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0532-0002900000-fc8081feb199f709b01c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fi0-4000090000-8fa5d02117228b181729 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-de6ec1b1a06abbcc5958 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0000900000-f40633a3526a735497bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fsi-0002900000-5e7b840d8437f9186179 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-2044700754efd0621641 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-0000900000-911dd86ae5c0bdca5d32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001l-1007900000-f743d551aba4b82eba9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000t-0000900000-cba3160c89e54b95b896 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0001900000-1ee36a966ad8e9edcfe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fu-0009200000-dab71ea1b7640d7f7ced | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-c37f5aca625bfa99356d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000900000-1cd12c34233dbf652cba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kbr-0009500000-db89c90b7974bbbce9bc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 168569 |
|---|
| PubChem Compound ID | 131752733 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|