| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:56:44 UTC |
|---|
| Update Date | 2016-11-09 01:20:51 UTC |
|---|
| Accession Number | CHEM033121 |
|---|
| Identification |
|---|
| Common Name | Cyclocalopin A |
|---|
| Class | Small Molecule |
|---|
| Description | Cyclocalopin A is found in mushrooms. Cyclocalopin A is a bitter principle isolated from Boletus calopus and other Boletus species. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
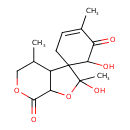
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H20O6 |
|---|
| Average Molecular Mass | 296.316 g/mol |
|---|
| Monoisotopic Mass | 296.126 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2',6-dihydroxy-2',4,4'-trimethyl-2',3'a,4',5',7',7'a-hexahydrospiro[cyclohexane-1,3'-furo[2,3-c]pyra]-3-ene-5,7'-dione |
|---|
| Traditional Name | 2',6-dihydroxy-2',4,4'-trimethyl-3'a,4',5',7'a-tetrahydrospiro[cyclohexane-1,3'-furo[2,3-c]pyra]-3-ene-5,7'-dione |
|---|
| SMILES | CC1COC(=O)C2OC(C)(O)C3(CC=C(C)C(=O)C3O)C12 |
|---|
| InChI Identifier | InChI=1S/C15H20O6/c1-7-4-5-15(12(17)10(7)16)9-8(2)6-20-13(18)11(9)21-14(15,3)19/h4,8-9,11-12,17,19H,5-6H2,1-3H3 |
|---|
| InChI Key | OGOHSCJKRSLFLO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as furopyrans. These are organic polycyclic compounds containing a furan ring fused to a pyran ring. Furan is a five-membered aromatic ring with four carbon atoms and one oxygen atom. Pyran a six-membered heterocyclic, non-aromatic ring, made up of five carbon atoms and one oxygen atom and containing two double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Furopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Furopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furopyran
- Delta valerolactone
- Cyclohexenone
- Delta_valerolactone
- Oxane
- Pyran
- Furan
- Tetrahydrofuran
- Carboxylic acid ester
- Hemiacetal
- Ketone
- Lactone
- Cyclic ketone
- Secondary alcohol
- Oxacycle
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fs9-3790000000-166a838ffa0d70b3f4e8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-4609400000-f21611935bea9d468985 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0190000000-cf5479b7fdf4fc9d2552 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gxt-1290000000-21051aee4eba55c4d4a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-8950000000-07432cd13e4f5aa41f6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0090000000-ef9bcb7f7b88175d2b62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002k-0290000000-fc5b437754dcbca6db3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0109-3930000000-51aab0ce6f5c02830310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-bbad7b000f11c0ba3f72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1390000000-d580b86430a67ac25581 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9620000000-e7b2d32faacc394c3ca5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-b424237b05a2bd372d62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0390000000-c01ccd274e48e2c0db1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dm-3910000000-35b2871889ed66bbe3c4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039810 |
|---|
| FooDB ID | FDB019460 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24535988 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 44631735 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|