| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:55:52 UTC |
|---|
| Update Date | 2016-11-09 01:20:51 UTC |
|---|
| Accession Number | CHEM033100 |
|---|
| Identification |
|---|
| Common Name | N-(1-Deoxy-1-fructosyl)isoleucine |
|---|
| Class | Small Molecule |
|---|
| Description | N-(1-Deoxy-1-fructosyl)isoleucine is classified as a Natural Food Constituent (code WA) in the DFC. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
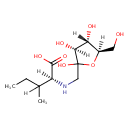
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-[(1-Carboxy-2-methylbutyl)amino]-1-deoxyfructose | HMDB | | (2S)-3-Methyl-2-({[(3S,4S,5R)-2,3,4-trihydroxy-5-(hydroxymethyl)oxolan-2-yl]methyl}amino)pentanoate | Generator |
|
|---|
| Chemical Formula | C12H23NO7 |
|---|
| Average Molecular Mass | 293.314 g/mol |
|---|
| Monoisotopic Mass | 293.147 g/mol |
|---|
| CAS Registry Number | 87304-79-8 |
|---|
| IUPAC Name | (2S)-3-methyl-2-({[(3S,4S,5R)-2,3,4-trihydroxy-5-(hydroxymethyl)oxolan-2-yl]methyl}amino)pentanoic acid |
|---|
| Traditional Name | (2S)-3-methyl-2-({[(3S,4S,5R)-2,3,4-trihydroxy-5-(hydroxymethyl)oxolan-2-yl]methyl}amino)pentanoic acid |
|---|
| SMILES | CCC(C)[C@H](NCC1(O)O[C@H](CO)[C@@H](O)[C@@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C12H23NO7/c1-3-6(2)8(11(17)18)13-5-12(19)10(16)9(15)7(4-14)20-12/h6-10,13-16,19H,3-5H2,1-2H3,(H,17,18)/t6?,7-,8+,9-,10+,12?/m1/s1 |
|---|
| InChI Key | VYGRYVGDPYFVCA-MFPOTZKYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as secondary alcohols. Secondary alcohols are compounds containing a secondary alcohol functional group, with the general structure HOC(R)(R') (R,R'=alkyl, aryl). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Secondary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary alcohol
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f96-9450000000-38c670b68836f898c5e8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-000i-9211047000-e147caad4625f59f07ff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-2590000000-b61a7f71b860a4c8b3c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-5590000000-03977477e82d0f141cfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9200000000-7655e7c46203bf69312f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-3390000000-3be80b7b4839d18d3b3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-007p-3490000000-630f2f12ad12ad64018d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-3f74bd9c8f86c0578609 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0090000000-4a6f0a1ac40e30ceb642 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00l7-5890000000-414b1095029c4877a088 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9300000000-77911d2de1b463705303 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-0290000000-740651f141d35ab75c0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-2890000000-3709f59ed92a9aa992ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9300000000-559d7ad36f269ce1f352 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039780 |
|---|
| FooDB ID | FDB019430 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014874 |
|---|
| ChEBI ID | 173136 |
|---|
| PubChem Compound ID | 131752725 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|