| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:55:43 UTC |
|---|
| Update Date | 2016-11-09 01:20:51 UTC |
|---|
| Accession Number | CHEM033097 |
|---|
| Identification |
|---|
| Common Name | Orcein |
|---|
| Class | Small Molecule |
|---|
| Description | Orcein, also archil, orchil, lacmus and C.I. Natural Red 28, are names for dyes extracted from several species of lichen, commonly known as "orchella weeds", found in various parts of the world. A major source is the archil lichen, Roccella tinctoria. Orcinol is extracted from such lichens. It is then converted to orcein by ammonia and air. In traditional dye-making methods, urine was used as the ammonia source. If the conversion is carried out in the presence of potassium carbonate, calcium hydroxide, and calcium sulfate (in the form of potash, lime, and gypsum in traditional dye-making methods), the result is litmus, a more complex molecule. The manufacture was described by Cocq in 1812 and in the UK in 1874. Edmund Roberts noted orchilla as a principal export of the Cape Verde islands, superior to the same kind of "moss" found in Italy or the Canary Islands, that in 1832 was yielding an annual revenue of $200,000.: pp.14, 15 Commercial archil is either a powder (called cudbear) or a paste. It is red in acidic pH and blue in alkaline pH. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
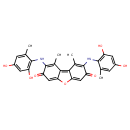
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| PAcein | MeSH | | C.I. natural red 28 | HMDB | | NSC 610930 | ChEBI | | Orcein | MeSH |
|
|---|
| Chemical Formula | C28H24N2O7 |
|---|
| Average Molecular Mass | 500.499 g/mol |
|---|
| Monoisotopic Mass | 500.158 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4,12-bis[(2,4-dihydroxy-6-methylphenyl)amino]-3,13-dimethyl-8-oxatricyclo[7.4.0.0²,⁷]trideca-1,3,6,9,12-pentaene-5,11-dione |
|---|
| Traditional Name | 4,12-bis[(2,4-dihydroxy-6-methylphenyl)amino]-3,13-dimethyl-8-oxatricyclo[7.4.0.0²,⁷]trideca-1,3,6,9,12-pentaene-5,11-dione |
|---|
| SMILES | CC1=CC(O)=CC(O)=C1NC1=C(C)C2=C3C(OC2=CC1=O)=CC(=O)C(NC1=C(O)C=C(O)C=C1C)=C3C |
|---|
| InChI Identifier | InChI=1S/C28H24N2O7/c1-11-5-15(31)7-17(33)25(11)29-27-13(3)23-21(9-19(27)35)37-22-10-20(36)28(14(4)24(22)23)30-26-12(2)6-16(32)8-18(26)34/h5-10,29-34H,1-4H3 |
|---|
| InChI Key | VPEASJIRGSVXBF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzofurans. These are organic compounds containing a benzene ring fused to a furan. Furan is a five-membered aromatic ring with four carbon atoms and one oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzofuran
- P-aminophenol
- O-aminophenol
- Aminotoluene
- Aniline or substituted anilines
- Resorcinol
- M-cresol
- Aminophenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Toluene
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Heteroaromatic compound
- Secondary ketimine
- Furan
- Azomethine
- Cyclic ketone
- Ketone
- Ketimine
- Oxacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-0632920000-123dd4ad3a506a96a39d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00fs-2330029000-852e97f1e2e24eb53623 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000190000-36b73a203923997d9ecc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0225790000-14fd8705726174ab45bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-1904400000-ab7844f6af08a959f022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-04502af16d5e9088017c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0001900000-a135eb875b7008ed9040 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-3900300000-0ede7f8faee4e358d05f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000090000-8aee1d79240e4c0d58aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0202490000-8255fed7cac1bd77a92b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ei-0305900000-cf3c7de19607cd988dc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0001900000-93cbe327dbf43898f8ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000900000-90727d3b11cb75be3caf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1009400000-742fe1b2998d483ad751 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Orcein |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 72685 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|