| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:54:29 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033066 |
|---|
| Identification |
|---|
| Common Name | 4-O-Methyl-a-D-glucosyl-(1->2)-b-D-xylosyl-(1->4)-D-xylose |
|---|
| Class | Small Molecule |
|---|
| Description | 4-O-Methyl-a-D-glucosyl-(1->2)-b-D-xylosyl-(1->4)-D-xylose is found in cereals and cereal products. 4-O-Methyl-a-D-glucosyl-(1->2)-b-D-xylosyl-(1->4)-D-xylose is from oat hull hemicelluloses. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
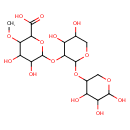
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-O-Methyl-a-D-glucopyranosyl-(1->2)-b-D-xylopyranosyl-(1->4)-D-xylose | HMDB | | 6-({4,5-dihydroxy-2-[(4,5,6-trihydroxyoxan-3-yl)oxy]oxan-3-yl}oxy)-4,5-dihydroxy-3-methoxyoxane-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C17H28O15 |
|---|
| Average Molecular Mass | 472.395 g/mol |
|---|
| Monoisotopic Mass | 472.143 g/mol |
|---|
| CAS Registry Number | 10365-86-3 |
|---|
| IUPAC Name | 6-({4,5-dihydroxy-2-[(4,5,6-trihydroxyoxan-3-yl)oxy]oxan-3-yl}oxy)-4,5-dihydroxy-3-methoxyoxane-2-carboxylic acid |
|---|
| Traditional Name | 6-({4,5-dihydroxy-2-[(4,5,6-trihydroxyoxan-3-yl)oxy]oxan-3-yl}oxy)-4,5-dihydroxy-3-methoxyoxane-2-carboxylic acid |
|---|
| SMILES | COC1C(O)C(O)C(OC2C(O)C(O)COC2OC2COC(O)C(O)C2O)OC1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C17H28O15/c1-27-11-8(21)10(23)16(32-13(11)14(24)25)31-12-6(19)4(18)2-29-17(12)30-5-3-28-15(26)9(22)7(5)20/h4-13,15-23,26H,2-3H2,1H3,(H,24,25) |
|---|
| InChI Key | XZGRJCXNJVJWKJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Glycosyl compound
- O-glycosyl compound
- Pyran
- Oxane
- Secondary alcohol
- Hemiacetal
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Monocarboxylic acid or derivatives
- Ether
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fu-7514900000-15092d24635160d900da | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-4901167000-120cd9f7bb254b2e059b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_31) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("4-O-Methyl-a-D-glucosyl-(1->2)-b-D-xylosyl-(1->4)-D-xylose,3TBDMS,#31" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsi-0960400000-06b90c11e2c6be5391a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0940000000-528b12f3d590a35e5994 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2920000000-84e657cdc5a0407325b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-2962500000-fcb397ff970921af8571 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-2941000000-926eacd895de9f8e2e3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0btd-4930000000-406a579b283adfd9305f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0101900000-cda482ec9950ad6f1cd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ac1-8726900000-4b5959b76fc926f3d73a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-9702000000-b2a0276f3a8c22d047d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0100900000-38c862b9e36d0c422dbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0awc-4924400000-2c50c2cc3e7dfdd97092 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015d-9503000000-b07275fb49b0e14f50b7 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039742 |
|---|
| FooDB ID | FDB019388 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 168540 |
|---|
| PubChem Compound ID | 78382737 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|