| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:54:15 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033060 |
|---|
| Identification |
|---|
| Common Name | Pipereicosalidine |
|---|
| Class | Small Molecule |
|---|
| Description | Pipereicosalidine is an alkaloid from fruits of Piper retrofractum (Javanese long pepper). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
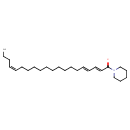
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C25H43NO |
|---|
| Average Molecular Mass | 373.615 g/mol |
|---|
| Monoisotopic Mass | 373.334 g/mol |
|---|
| CAS Registry Number | 145237-20-3 |
|---|
| IUPAC Name | (2E,4E,16Z)-1-(piperidin-1-yl)icosa-2,4,16-trien-1-one |
|---|
| Traditional Name | (2E,4E,16Z)-1-(piperidin-1-yl)icosa-2,4,16-trien-1-one |
|---|
| SMILES | CCC\C=C/CCCCCCCCCC\C=C\C=C\C(=O)N1CCCCC1 |
|---|
| InChI Identifier | InChI=1S/C25H43NO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19-22-25(27)26-23-20-18-21-24-26/h4-5,16-17,19,22H,2-3,6-15,18,20-21,23-24H2,1H3/b5-4-,17-16+,22-19+ |
|---|
| InChI Key | MKEWSYYOWWKTAK-AEPHKGLBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylpiperidines. N-acylpiperidines are compounds containing an N-acyethanolamine moiety, which is characterized by an acyl group is linked to the nitrogen atom of a piperidine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Piperidines |
|---|
| Sub Class | N-acylpiperidines |
|---|
| Direct Parent | N-acylpiperidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-piperidine
- Tertiary carboxylic acid amide
- Carboxamide group
- Azacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01u9-6972000000-4db8128dca6ef14e04b1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-3119000000-6026003efecfd9e1951c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9472000000-5a3c0880878113a7092f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9130000000-e542377d2230612a70c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-14a8fedd51efe5d057dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00e9-7349000000-fa2594e43f117e2f60c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9100000000-189da8e97d2e835d1e9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-f3ba5b47bb6913d11ff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0329000000-b1d67ad5e29807fb6aa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9326000000-df616456d58eae815885 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-6059000000-29fe0366226043a6cbf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9011000000-52911e4bec66c69f3cb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9000000000-d20c7cf71898dc643133 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039738 |
|---|
| FooDB ID | FDB019380 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057068 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777364 |
|---|
| ChEBI ID | 174961 |
|---|
| PubChem Compound ID | 101634668 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|